Phenospot
The behaviour and function of cells within our bodies are coordinated by signalling molecules that are passed between cells. To properly understand these complex signalling events, we need tools that allow us to observe signalling and measure the corresponding cellular response quantitatively and in real time. Current tools can either measure signalling from a large number of cells at a single time point, or they have to destroy the cells in order to analyse them.
We are developing a fundamentally different imaging technique, which we term "Phenospot" and which is based on optical devices called photonic resonators that allow us to identify which cells are communicating, when, how and with whom. We have already demonstrated the potential of the technology but are now optimising its performance to address a range of questions critical for health including immunity, cancer progression and wound healing and provide new ways to help treat disease, for example to develop better vaccines.
This is a highly interdisciplinary project involving collaboration between the Departments of Physics (Krauss), Electronic Engineering (Johnson), HYMS (Kaye) and Biology (Hitchcock, Kent, Southgate).
J. J, Colás, I. S. Hitchcock, M. Coles, S. Johnson, S. T. F. Krauss, Quantifying single-cell secretion in real time using resonant hyperspectral imaging, Proc. Natl. Acad. Sci. 115, 13204 (2018)
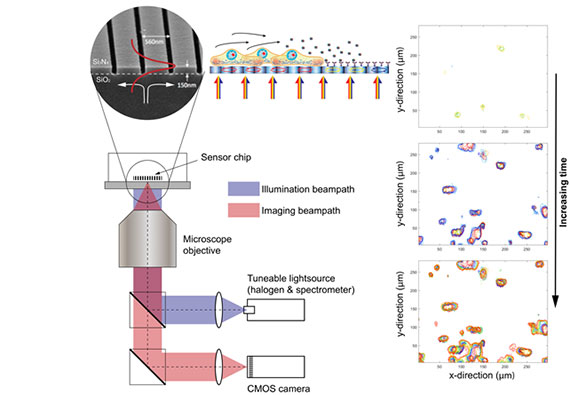
The Phenospot sensor chip, here a 1D grating, can be implemented on a standard microscope simply by changing the illumination beampath to a wavelength-tunable source. Proteins secreted from individual cells local to the sensor surface bind to surface-immobilised antibodies leading to a local change in refractive index and, in turn, a shift in the resonant wavelength of the sensor chip. By recording the shift in resonance wavelength over time, it is possible to map the spatial distribution, onset and kinetics of protein secretion from single cells.
Read more about Professor Thomas Krauss' journey on making sci-fi health technology a reality for everybody on the University's Catalyst for Change page.
