Cancer@York
Cancer research at York spans a range of cancer types and processes, as profiled below.
Contact us
York Biomedical Research Institute
ybri@york.ac.uk
Department of Biology, Wentworth Way, University of York, York, YO10 5NG
X
Jack Birch Unit
The Jack Birch Unit (JBU) has an internationally recognised reputation for human urothelial cancer biology, offering an advanced experimental ex vivo platform for human urinary tract epithelial cells and tissues, supported by state-of-the-art analytical and data informatics approaches and an NHS REC-approved urothelial research tissue bank. The JBU is the flagship research unit for local charity, York Against Cancer, who provide core programme funding and was named after Jack Birch OBE, the first Chairperson of York Against Cancer and former Lord Mayor of York.
Key Researchers
Professor Jenny Southgate is Professor of Molecular Carcinogenesis and current Director of the Jack Birch Unit, as well as theme lead for Molecular & Cellular Medicine in the York Biomedical Research Institute. Her research interests focus on mechanisms that regulate cell proliferation versus differentiation in normal epithelial tissue homeostasis to bring insight to the selection pressures that drive cancer development and evolution along different pathways.
Dr Simon Baker works on the origins of bladder cancer, focussing on the control of the APOBEC family of anti-viral enzymes thought to cause the DNA damage that initiates carcinogenesis. Through understanding what causes APOBECs to damage the DNA of bladder cells, Simon seeks new approaches to preventing the disease. We recently published an article suggesting the induction of APOBECs and the initiation of bladder cancer could be driven by BK polyomavirus and this work forms the basis of our current Kidney Research UK and York Against Cancer funded research.
Dr Andrew Mason. The Mason Lab is a bioinformatics group researching human and avian cancers. Our broad aim is to use sequencing data to understand carcinogenesis and to stratify cancers in biologically and clinically relevant ways to derive more personalised treatments. Our research has two main areas: characterising human urothelial carcinoma, and understanding the impacts of endogenous retrovirus interactions in avian and human cancers. Our lab leads the bioinformatic analysis of bladder cancer in the Genomics England 100,000 Genomes Project.
Active funding: York Against Cancer, Kidney Research UK, MRC, Elixir-UK
Haematological Malignancies Research
The Centre for Blood Research (CBR) brings together expertise from experimental, clinical and epidemiological research areas to work on our understanding of blood cancers with the view to developing novel treatments for patients.
Within the CBR sits the Haematological Malignancies Research Network (HMRN) which collects and analyses all of the clinical data from patients with haematological malignancies and is led by Professor Eve Roman and Professor Alexandra Smith.
Key Researchers
Professor Dave Kent has research interests in cancer evolution, stem cell biology, cancer genetics and haematological malignancies. He is currently Deputy Head of Department (Research) in Biology.
Professor Ian Hitchcock studies the activity and function of cytokine receptors and their signalling in blood cancer cells. He is currently the Director of the CBR.
Professor Adele Fielding studies the experimental and clinical biology of acute lymphoblastic leukaemia (ALL) and is Chief Investigator for a number of clinical trials in the UK. Her laboratory research focuses on understanding the biology of ALL. She is currently the Clinical Lead for the CBR.
Dr Katherine Bridge is a cancer cell biologist whose laboratory focuses on Hypoxia Inducible Factors (HIFs) and aberrant transcription in malignant transformation. She established her group in 2021 with a fellowship from the Kay Kendall Leukaemia Fund, focusing on the molecular mechanisms underpinning context-specific activation of HIFs in haematopoietic stem cells and myeloid neoplasms. The Bridge Lab is highly collaborative with research modalities spanning biophysics, biochemistry, cell biology, multi-omics and clinical haematology to analyse how the chromatin interactome directs malignant transformation. The Bridge Lab also studies HIF function within breast cancer, with active grants held with both Dr Will Brackenbury and Dr Andrew Holding.
Dr William (Bill) Grey’s research focuses on the role of protein homeostasis in normal and malignant stem cell development. His team are studying the role of protein homeostasis in normal and malignant stem cell development across stem cell systems, with a principle focus on the transformation of healthy HSCs to malignant LSCs. He is funded by a MRC Career Development Award, a MRC National Mouse Genetics Network Director’s Fund, Leukaemia U.K., the Children’s Cancer and Leukaemia Group, the Lady Tata Trust and the European Haematology Association. His team also lead the UK patient-derived xenograft biobank in association with the Mary Lyon Centre at MRC Harwell.
Professor Dimitris Lagos’ main cancer research interests are the role of RNA (non-coding and regulatory) in cancer immune gene expression (projects on clear cell renal cell carcinoma and haematological malignancies); RNA immunotherapeutics; Tumour microenvironment; Immune checkpoint regulation; Natural products as a source of cancer immunotherapies; Novel omics (including long-read) and digital pathology platforms.
Funding from: CR-UK, MRC, Bill and Melinda Gates Foundation, and industry partners (STRM.bio, STEMBOND). Kay Kendall Leukaemia Fund, Blood Cancer UK, European Hematology Association, BBSRC, MRC, Canadian Stem Cell Network, Leukaemia UK, The Children’s Cancer and Leukaemia Group.
- Burt et al., BLOOD 2019
- Che et al., EMBO Reports 2022
- Foxler et al., EMBO Molecular Medicine 2018
- Grey et al., Hemasphere 2023
- Grey et al., Science Translational Medicine 2022
- Igarishi et al., Blood Advances 2023
- Lee-Six et al., Nature 2018
- Machado et al., Nature 2022
- Obro et al., Hemasphere 2020
- Shepherd et al., BLOOD 2018
- Wilmes et al., Science 2020
Breast Cancer Research
Key Researchers
Dr Will Brackenbury. Aberrant sodium channel/transporter expression in cancer cells may be related to the elevated tissue sodium levels reported in solid tumours, including breast cancer. Using 23Na-MRI, we have shown that this elevated sodium predominantly resides in the intracellular compartment, suggesting that altered plasma membrane transport routes, including VGSCs, may be an important mechanism underlying sodium accumulation in breast cancer. Using a combination of preclinical in vitro and in vivo models, machine learning, and imaging studies in cancer patients, we are currently investigating how expression of these channels is regulated and the potential utility of altered sodium as a novel imaging biomarker of malignancy.
Dr Andrew Holding's team studies the epigenetic mechanisms that enable different tissues to respond differently to the same steroids and how the regulation of this process changes between healthy and cancerous tissues. Understanding these processes is vital as in cancer the steroid hormone response is commonly hijacked, or altered, leading to it drive the disease. We achieve this aim through the use of state-of-the-art genomic and proteomic methods, including ChIP-seq, RIME and machine learning across breast, bladder and blood primary tissues. Our long-term goal is to apply this knowledge to bypass treatment resistance in breast cancer, improving the outcomes for patients. Research is funded through BBSRC, Wellcome and The Royal Society.
Funding from CRUK, BBSRC, MRC
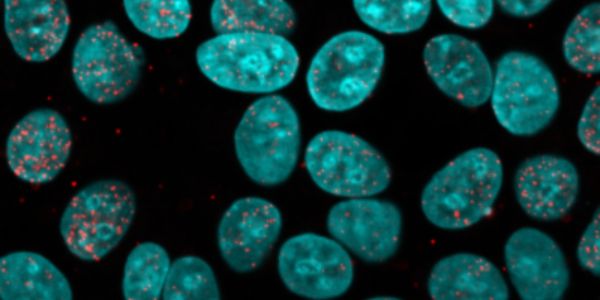
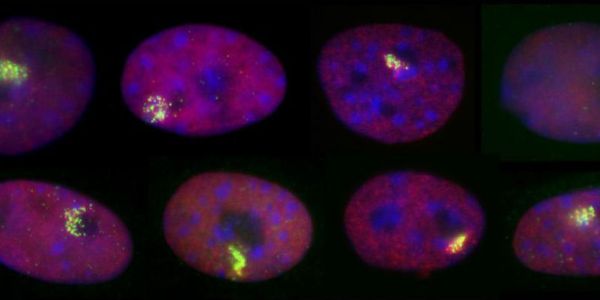
'Estrogen Receptor (Red) in the Nuclei (Blue) of ER+ Breast cancer cells'
Credit: Susanna Rose (Holding Lab)
Fundamental processes in cancer
Professor Dawn Coverley’s research interests lie in the spatial organisation and patterning of the mammalian cell nucleus, and its degeneration in human disease. Her team are focussed on the function of the CIZ1 protein in maintenance of chromatin through cellular transition states, and the effects of CIZ1 loss or corruption on accurate execution of change. An important aspect of this work is the identification of information with potential clinical application, and crucially in taking those opportunities through to exploitation and application.
Funding: MRC and Cizzle Biotech
Carcinogenesis and malignancy: Research snapshots
Contact us
York Biomedical Research Institute
ybri@york.ac.uk
Department of Biology, Wentworth Way, University of York, York, YO10 5NG
X