Dr William Grey
MRC Career Development Award Fellow
Visit Dr William Grey's profile on the York Research Database to:
- See a full list of publications
- Browse activities and projects
- Explore connections, collaborators, related work and more
Profile
Biography
Bill completed his PhD at King's College London with Dr Veronica Yu and Professor David Grimwade, studying the role of protein phosphorylation and degradation across stem cell systems, including embryonic, neural and haematopoietic systems. He continued studying protein biology as a postdoctoral fellow at The Francis Crick Institute with Professor Dominique Bonnet. Focusing on haematopoietic stem cells and acute myeloid leukaemia.
Bill was awarded a John Goldman Fellowship by Leukaemia UK, MRC career development award and a Children's Cancer and Leukaemia Group project grant to start his independent research programme at the University of York, working on protein homeostasis (so called "Proteostasis") in normal and malignant stem cells of the haematopoietic system.
During his time in London, Bill founded the London Stem Cell Network and maintains strong links with scientists across stem cell systems. Bill has been a Royal Society Westminster Fellow and worked with the Brilliant Club since 2012 to bring scientists closer to politicians, general public and most importantly primary and secondary education.
External links:
- ProteoStem homepage
- Leukaemia UK
- Children's Cancer and Leukaemia Group
- Lady Tata Trust
- The UK PDX BioBank (coming soon)
Lab members:
- Juliana Miranda (PDRA))
- Megan Guthrie (MRC-DiMeN PhD student)
- Anjum Kahn (MRC CARP partner & consultant haematologist)
- Zahra Masoumi (Senior Research Associate)
- William Critchley (PDRA)
- Kimrun Kaur (YGRS PhD student)
- Lauren Wheeler (MRes student)
- Daniel Davies (NC3Rs PhD student)
Alumni:
- Beatrice Rix (current: Crick PhD student, London)
- Adam Rogerson (current: Research Associate Zenker lab, Monash, Australia)
- Katie Trueman (current: Final year student, Cardiff University, Wales)
- Max Gregoryan (current: BBSRC DTP PhD student)
- Navin Shirodkar (current: BBSRC DTP PhD student, Sheffield)
Funders:





Research
Overview
Our research focuses on the role of protein homeostasis - so called “Proteostasis” - in healthy and malignant stem cell development. The predominant focus of the lab is the haematopoietic (blood) system and cancerous transformation to acute myeloid leukaemia (AML).
Projects
Haematopoiesis: the prototypical stem cell system to study proteostasis. Haematopoiesis is a crucial example of a stem cell hierarchy and represents the ideal system to investigate tissue homeostasis, age-related systemic failure and malignant transformation to cancer (Figure 1). Extensive studies have provided insights into the role of genetics, epigenetics, and the niche in both normal and malignant haematopoietic development. However, our understanding of protein translation, folding, modification and degradation – broadly defined as “proteostasis” – is in its infancy. Considering the dynamic nature of the proteome, both temporally and spatially, the regulation of healthy and malignant stem cells at the protein level remains an exciting novel area of research.
Acute Myeloid Leukaemia (AML) is a heterogeneous disease of the haematopoietic system with a dismal prognosis. Two-year survival rates are 5-15% in cytogenetically poor risk patients, underlining the critical need for new therapies. Current approaches of induction chemotherapy and epigenetic modifiers have been hampered by the significant plasticity in the Leukaemic Stem Cell (LSC) epigenome and are insufficient to substantially improve patient outcomes. LSCs are the origins of relapse in AML and show substantial plasticity from de novo disease through to relapse, where they drive a more aggressive disease.
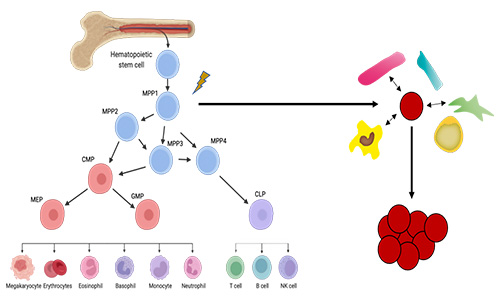
Figure 1. Illustration of the healthy haematopoietic system (left) and the malignant transformation to produce leukaemic cells, both LSCs and blasts, with LSCs surrounded by supportive niche cells (red, right).
Studying proteostasis in haematopoietic stem cells (HSCs). We study the proteome in HSCs, with a particular focus on expansion of HSCs for transplantation. We have demonstrated that loss of the proteostasic regulators CKS1 and CKS2 blocks the ability of HSCs to reform the haematopoietic system upon transplantation (Hemasphere 2023), and by carrying out paired proteomics and transcriptomics demonstrated that there is a disconnect between the proteome and transcriptome in HSCs (Figure 2). An axis we are continuing to study across haematopoiesis and stress responses.
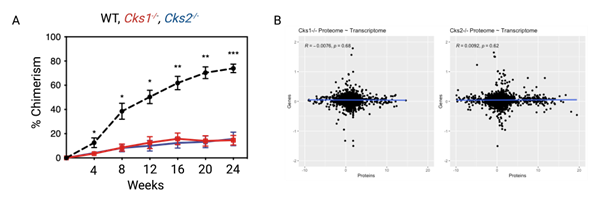
Figure 2. A. Peripheral blood chimerism of WT recipient mice transplanted with WT (black), Cks1-/- (red) and Cks2-/- (blue) long–term HSCs. B. Correlation between differentially expressed proteins (x-axis) and genes (y-axis) in Cks1-/- and Cks2-/- HSCs.
HSCs require tightly regulated protein kinase signalling cascades, historically most well understood through study of instructive signals (cytokines) from the bone marrow microenvironment. We studied the functional kinome of human HSCs derived from umbilical cord blood to reveal the receptor tyrosine kinase, RET, is a functional target on the cell surface of HSCs which governs a multitude of signalling pathways important for stem cell maintenance and expansion (Blood 2020, Figure 3.)
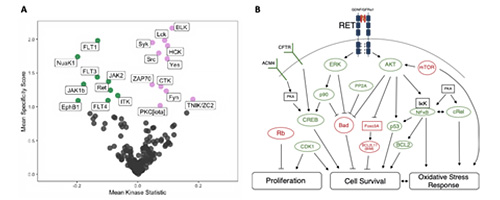
Figure 3. A. Differentially active kinases in CD34+CD38- (Green) versus CD34+CD38+ (Violet) umbilical cord blood derived haematopoietic stem and progenitor cells. B. Key signalling pathways downstream of GDNF/GFRa1-dependent activation of RET on HSCs. Green proteins are hyperactive, red proteins are hypoactive post-GDNF/GFRa1 treatment.
Targeting proteostatic regulators in AML. We study the role of protein homeostasis in AML using a variety of novel inducible mouse models (Cancer Cell 2016) and primary patient samples (Science Translational Medicine 2022). By focussing on LSCs and their underlying biology, we design new methods for targeting drug resistance and relapse to improve overall survival in AML (Figure 4).
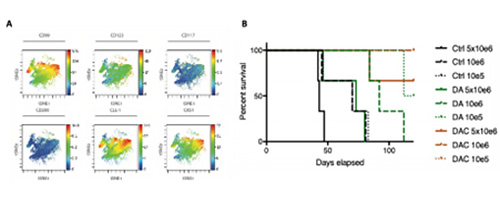
Figure 4. A. Example tSNE plot demonstrating AML leukaemic stem cell subpopulations and co-expression of a novel target (CKS1). B. Overall survival of patient derived xenograft models treated with novel chemotherapeutic approaches.


