Vaccine targets for animal African trypanosomiasis
Translating the protective effects of antibodies to invariant T. vivax antigens from mouse to cattle
Context
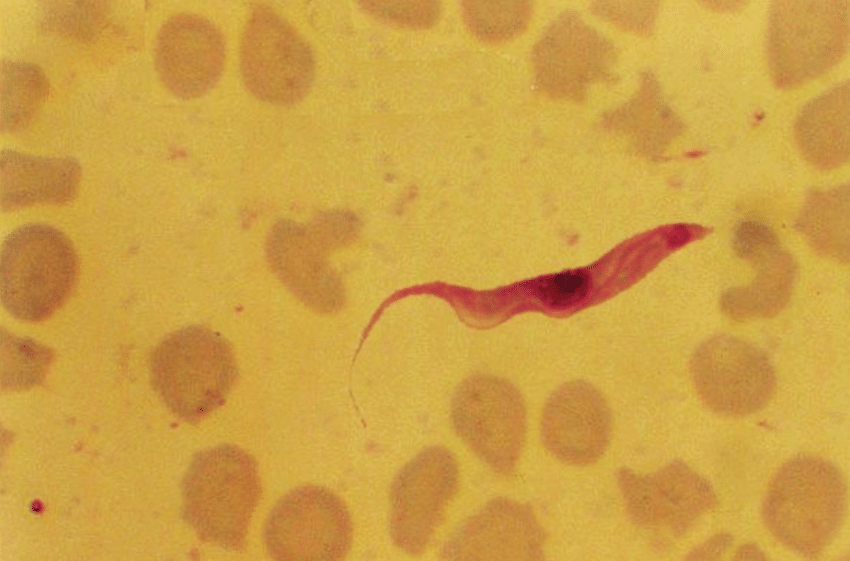
Animal African Trypanosomiasis (AAT) has a significant impact on animal agriculture, principally in sub-Saharan Africa, by threatening the livelihood of subsistence farmers and food security in endemic countries. Trypanosoma vivax and Trypanosoma congolense are the primary causes of this disease in sub-Saharan Africa, and the infection of cattle by these parasites is responsible for enormous direct economic losses estimated at hundreds of millions of dollars annually. While vaccination would be an ideal solution to manage AAT, effective vaccines against African trypanosomes were considered unachievable, primarily due to the sophisticated system of antigenic variation employed by these pathogens to elude the host immune response.
To identify vaccine candidates for T. vivax we have used a systematic genome-led reverse vaccinology approach to test antigenically invariant proteins on the surface of T. vivax blood-stage parasites for their ability to protect against infection when used as subunit vaccines. To achieve this, we have compiled libraries of soluble recombinant proteins corresponding to the entire ectodomains of cell surface and secreted proteins which, importantly, were expressed in mammalian cells to increase the chances that structurally-critical post-translational modifications were added. Using this approach, we have identified two promising candidates for T. vivax. In T. vivax, two proteins named V23 and V31 were identified (Autheman et al.). When these proteins were used in a protein-in-adjuvant vaccine formulation using QuilA in mice, these proteins are capable of eliciting sterile protection. We have shown that antibodies are a major immune mechanism because immune sera against V23 are capable of eliciting protection in passive transfer experiments.
Research into the V23 candidate is currently more advanced and we have shown that it has a subcellular localisation that is restricted to the interface of where the flagellum adheres to the cell body of the parasite. By selecting a panel of monoclonal antibodies to the extracellular region of V23, we have demonstrated we can confer protection to infection when passively transferred. While our initial experiments were done using a mouse IgG1 isotype, we observed that the protective effects were far more potent when the isotype of the antibody was changed to an IgG2a; this almost certainly is because IgG2a, unlike IgG1 isotypes, can recruit immune effectors such as complement and bind activating Fc receptors. By individually mutating antibody effector binding sites on protective recombinant antibodies, complement recruitment was found to be a major protective mechanism (Autheman et al). The purpose of the current project is to translate the protective effects of antibodies to invariant T. vivax from the mouse model to cattle.
Aims and Objectives
- Establish an experimental platform in cattle to test T. vivax vaccine candidates.
- Quantitating protective antibody titres in mice and antigenicity studies in cattle.
- Subunit vaccination trial for T. vivax in cattle.
Related links
For further information please visit the project website
Professor Gavin Wright, Department of Biology
Principal Investigator
Professor Gavin Wright, Department of Biology
Co-Investigator
Professor Liam Morrison, Roslin Institute
Bill and Melinda Gates Foundation
Clinvet
Autheman-D, Crosnier-C, Clare-S, Goulding-DA, Brandt-C, Harcourt-K, Tolley-C, Galaway-F, Khushu-M, Ong-H, Romero-Ramirez-A, Duffy-CW, Jackson-AP, Wright-GJ. “An invariant Trypanosoma vivax vaccine antigen inducing protective immunity.” Nature 2021 595 p96.
Related links
For further information please visit the project website