Extending meta-analysis so that health benefits are used to prioritise research and implementation efforts
Posted on Wednesday 2 December 2015
Decisions about undertaking further research, and at what point evidence should be implemented, are important considerations when prioritising research and health policy. Meta-analysis can assist in this process. This statistical tool combines the results from a number of studies to provide a single pooled result. The results of standard meta-analysis can be extended to assess the potential health benefits of gathering additional evidence and of implementing the findings, in a way that cannot be achieved by examining statistical significance alone.
The uncertainty associated with whether an intervention is effective is indicated by the results of meta-analysis. By combining the estimated relative measure of effect on health (such as the odds ratio for mortality) with estimates of baseline risk and incidence of ill-health, it is possible to estimate the health consequences of this uncertainty. These consequences are the amount of health that could be lost from adopting a less effective intervention based upon current evidence, and represent the best possible health benefits of conducting further research.
Health outcomes can also be improved by ensuring that the findings of existing research are better adopted within clinical practice. Combining the central estimate of relative health effect from meta-analysis with estimates of baseline ill-health risk and incidence, allows the expected health benefits of implementing research findings to be estimated.
To illustrate how the potential benefits of further research versus implementing current research findings can evolve, the figure below shows three points in the sequence of clinical trials that investigated early treatment for blood clots (thrombolysis) using an enzyme based drug (streptokinase) (versus no thrombolysis) after a heart attack. In this example, the expected benefits of implementing the findings of existing research quickly exceeded the potential benefits of further research.
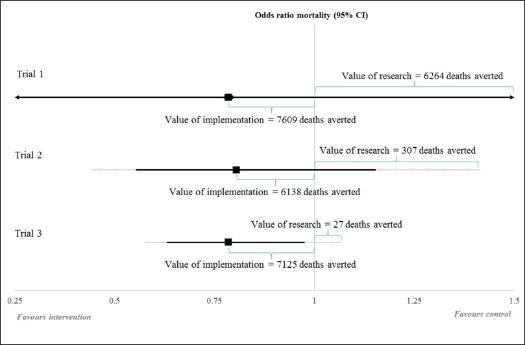
However, early implementation of new interventions, based on the balance of evidence, might not always be appropriate if their widespread use means that further research (for example, with adequate control) becomes impossible or more costly. In these cases, it may be better to delay implementing research findings until the benefits of further research are secured for future patient groups.
Further information
The paper "How to estimate the health benefits of additional research and changing clinical practice", authored by Karl Claxton, Susan Griffin, Claire McKenna, and Hendrik Koffijberg, is published in the British Medical Journal. To read, please visit: www.bmj.com/content/351/bmj.h5987
This research originated as a commissioned report to inform 'The Patient-Centered Outcomes Research Institute' (PCORI) in the United States and was presented at the 'PCORI Methodology Workshop for Prioritizing Specific Research Topics'. For the full report, please see: CHE Research Paper 83 (PDF
, 1,068kb)
Further information can be found on the University of York news page: www.york.ac.uk/news-and-events/news/2015/research/health-meta-analysis-tool/
