Enzymes enable selective preparation of medicinally important chiral sulfur compound
Posted on Monday 19 January 2026
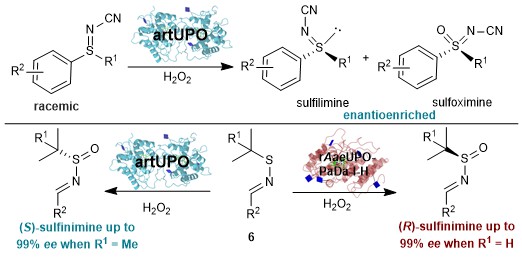
Unspecific Peroxygenase enzymes – ‘UPOs’ for short – are an important class of enzyme, which in nature are secreted by fungi to detoxify their environment.
In a recent study published in Nature Communications, Dr Jiacheng Li, Dr Benjamin Melling and coworkers, working with Gideon Grogan and Will Unsworth, harnessed UPOs for the selective preparation of a range of medicinally important chiral sulfur compounds.
UPOs work by enabling the selective oxidation of small molecules. In this study their application in the enantioselective oxidation of various types of sulfur-containing molecules is reported for the first time. Classical oxidation methods typically make use of hazardous and/or toxic reagents. In contrast, UPO mediated oxidation reactions operate under benign conditions, use non-toxic reagents and can be performed in water, offering a much safer and more sustainable approach. The chiral environment provided by the UPO also allows products to be selectively formed as single enantiomers, or with a single ‘handedness’.
This work adds to a growing legacy of important and practical UPO-mediated oxidation reactions to emerge from a successful ongoing collaboration between the laboratories of Prof Gideon Grogan and Dr Will Unsworth in the Department of Chemistry, University of York.
This work was funded by the EPSRC and Syngenta.
Notes to editors:
This work has been published in Nature Communications
