New spectroscopic approach reveals how key tuberculosis drug acts inside living bacteria
Posted on Thursday 18 December 2025
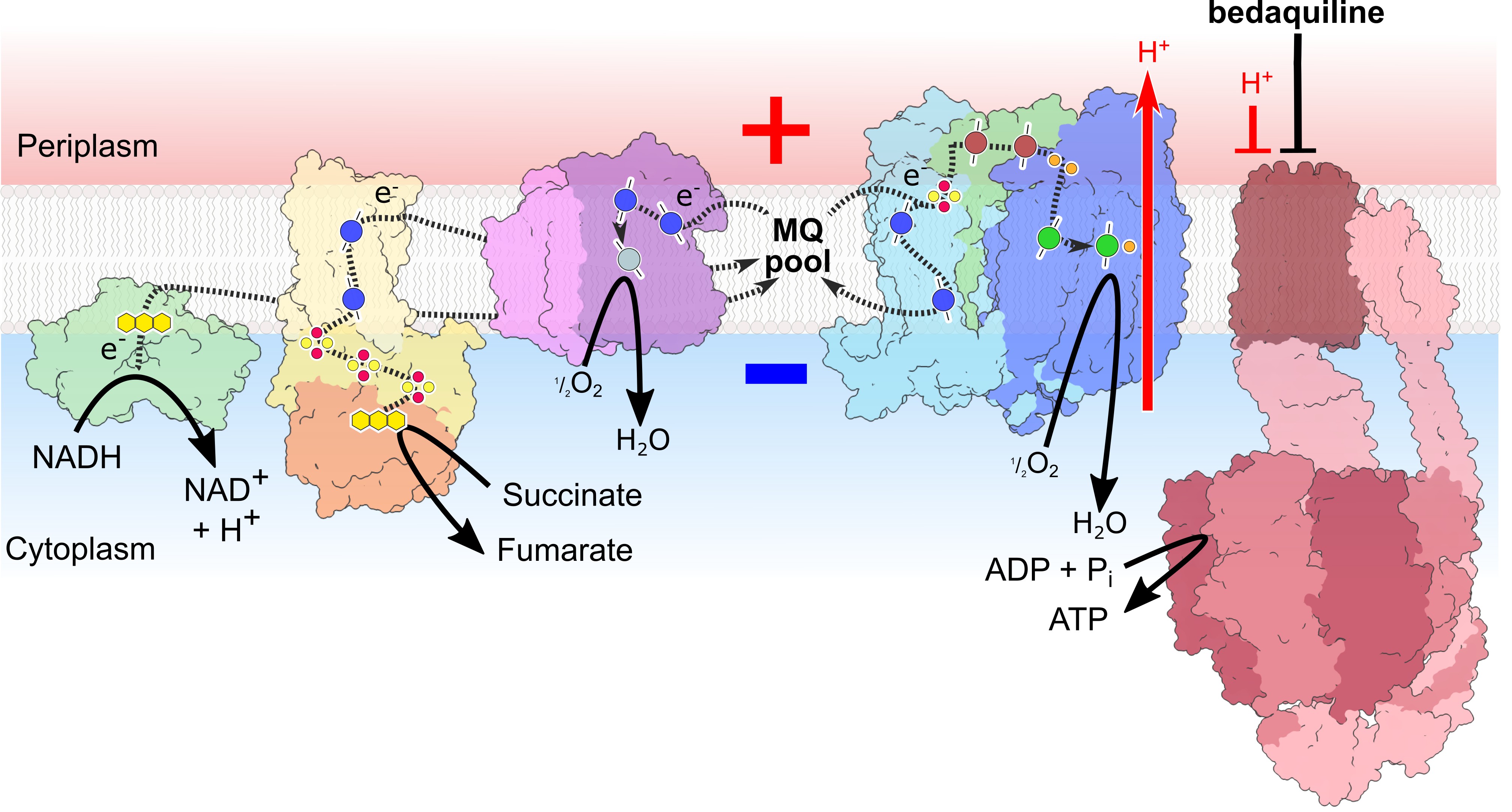
Tuberculosis is the World’s deadliest infectious killer. New research from Professor Jamie Blaza’s team in the Department of Chemistry has uncovered the precise bioenergetic mechanism by which the tuberculosis drug bedaquiline acts inside living cells, using a novel remission spectroscopic approach to observe electron flow in the living pathogen in real time.
When approved, bedaquiline was the first new drug against tuberculosis in over four decades, being effective even against drug-resistant forms of the pathogen. Bedaquiline binds to the enzyme ATP synthase, which is powered by proton flow through the enzyme much like water through a turbine. The team at York have now demonstrated that rather than binding to the enzyme and disrupting it into a ‘leaky state’, bedaquiline instead interacts tightly with ATP synthase, causing a block to proton flow, which then results in a thermodynamic ‘traffic jam’ within the bioenergetic system of the tuberculosis bacterium.
The work was led by two PhD students, Dr Suzy Harrison and Dr Rowan Walters, together with Dr Morwan Osman, a research associate, working in the laboratory of UKRI Future Leader Fellow, Jamie Blaza with key collaborations in Otago, New Zealand. It is published in Nature Communications.
Notes to editors:
This work has been published in the the journal Nature Communications.
