Fluorinated Bacterial Sugar Stops Bacteria Swimming
Posted on Tuesday 4 November 2025
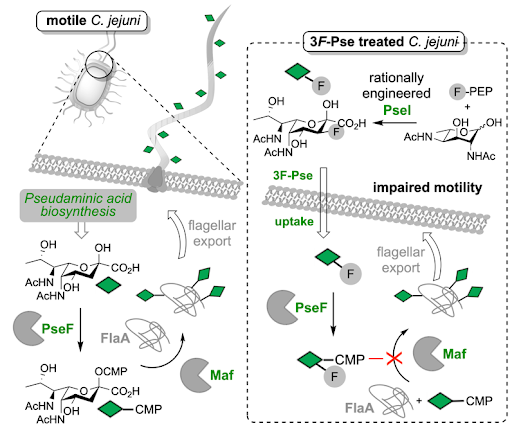
Many bacteria, including pathogenic species such as Campylobacter jejuni, the main cause of foodborne disease in the developed world, produce long tail-like structures called flagella which allow them to swim and infect host systems. A complex pathway has evolved in these bacteria to assemble flagella. Disrupting this pathway therefore offers the potential to stop flagella from forming and hence the bacteria from swimming.
Although the mechanism of flagella production is not yet completely understood, it is known that a bacterial sugar called pseudaminic acid plays a vital role. Pseudaminic acids aid the export of the flagella building blocks and are also presented on the surface of these structures.
PhD student James Jeffries working in the lab of Dr Martin Fascione from the Department of Chemistry, in collaboration with Prof Gavin Thomas from the Department of Biology, created a pseudaminic acid mimic that can potentially bind an enzyme involved in the decoration of flagella with pseudaminic acid sugars, inhibiting flagella assembly. The team’s approach involved a design of a fluorinated version of the sugar which may inhibit a key biosynthetic enzyme, preventing it from functioning.
Importantly, the synthesis of this fluorinated inhibitor was optimised using a mixture of chemical and enzymatic steps, and deploying an enzyme mutated by rational design to dramatically improve its compatibility with the unnatural fluorine-containing molecule.
Dr Martin Fascione said “The discovery of this new approach to impair bacterial motility through the use of fluorine containing sugars, highlights an ‘Achilles heel’ in some pathogenic gut bacteria which could be targeted through development of the next generation therapeutics with potential to treat multi-drug resistant infectious diseases. We are currently working towards future developments in this innovative area.”
Notes to editors:
This research has been published in Angewandte Chemie.
