Electrostatic Ion Binding Reshapes Light Absorption
Posted on Tuesday 9 December 2025
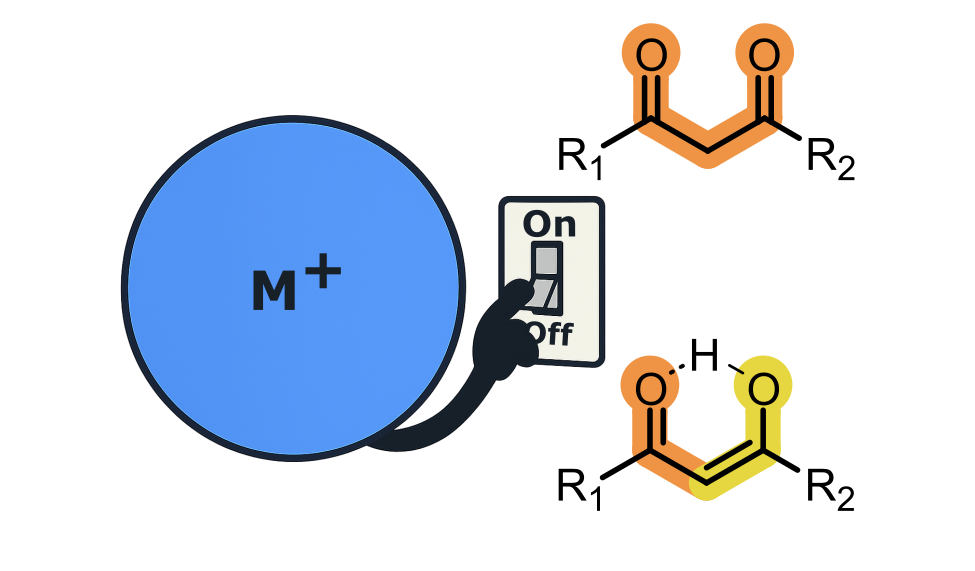
Researchers in the Department of Chemistry have used a combination of laser spectroscopy and quantum chemistry to investigate how simple metal ions, such as those found in table salt, can affect the light-absorption properties of avobenzone. Avobenzone is a common sunscreen molecule but is also a prototype “keto-enol” molecule. The keto-enol motif underpins much of synthetic organic chemistry and over recent years, light has been used to control this reactivity.
Published in the Journal of the American Chemical Society, the researchers found that metal ion binding reverses the stability of the keto and enol structures and hence dramatically changes the reactive events that follow light absorption in such systems. The work demonstrates how the electrostatic field exerted by a simple ion can change a molecule it binds to - this is important given how widespread keto-enol structures are in chemistry.
The results also have implications for the development and use of sunscreens, since Avobenzone is a widely used UVA filter molecule. Metal ion binding to Avobenzone, which can happen when a sunscreen formulation comes into contact with human skin or the sea, will reduce its photostability.
Post-doctoral researchers Dr Sarah Wilson and Dr Cate Anstöter jointly led the project, which was partly conducted at the FELIX Free Electron Laser for Infrared eXperiments at Radboud University in the Netherlands.
Find out more at https://pubs.acs.org/doi/10.1021/jacs.5c12521
Notes to editors:
This work has been published in the Journal of the American Chemical Society
