Mammalian glycosylation and human disease
Understanding the roles of carbohydrates, and the enzymes that make and degrade them, in cell physiology and human disease.
Lead researcher: Dr Lianne Willems, Department of Chemistry
Cell-surface proteins and lipids are decorated with a wide variety of carbohydrate structures, named glycans, that play central roles in mammalian biology. They are key regulators of, for instance, cell recognition and signalling events. At the same time, glycan interactions are often hijacked by pathogens to enter host cells, while undesirable changes in a cell’s glycan levels can cause pathological conditions such as tumour metastasis or muscular dystrophy (Figure 1).
The complex and diverse nature of glycan structures mirrors the enormous diversity of cellular enzymes that are involved in synthesising and modifying the many carbohydrate structures in the endoplasmic reticulum (ER) and Golgi apparatus. The complexity of these biosynthetic pathways makes it a challenging task to decipher the exact roles of individual enzymes and glycan structures in cells, knowledge that is essential if we are to understand how malfunctioning leads to disease.
Dr Willems’ research focuses on developing novel chemical tools to study some of these glycans and the enzymes responsible for their biosynthesis. These tools are applied to a range of biochemical, structural and cellular studies to enhance our fundamental understanding of glycobiology. This knowledge also helps us better understand how abnormal glycan levels or malfunctioning of specific carbohydrate-active enzymes leads to disease, opening up new avenues for therapeutic intervention.
Another line of Willems’ research focuses on the degradation of glycoconjugates in the lysosome. Defects in the activity of these enzymes causes accumulation of their substrates, which leads to lysosomal storage disorders. Again, chemical tools are used to probe the enzymes of interest and provide insights into the molecular and cellular mechanisms of disease.
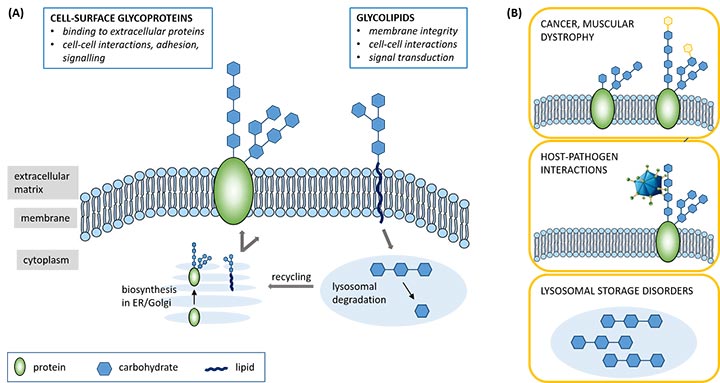
Figure 1: Carbohydrates, linked to proteins or lipids, decorate the surfaces of our cells. They play key roles in both ‘healthy’ cellular functioning (A) and in disease pathology (B).
Key papers
- Huxley, K. E. and Willems, L. I. Chemical reporters to study mammalian O-glycosylation, Biochem. Soc. Trans. 2021, 49 (2), 903-913.
- Zhu, Y. et al., J. Am. Chem. Soc. 2020, 142 (37), 15729–15739.
- Willems, L. I. et al. Potent and selective activity-based probes for GH27 human retaining α-galactosidases, J. Am. Chem. Soc. 2014, 136, 11622
- Willems, L. I.; Li, N.; Florea, B. I.; Ruben, M.; van der Marel, G. A.; Overkleeft, H. S. Triple bioorthogonal ligation strategy for simultaneous labeling of multiple enzymatic activities, Angew. Chem. Int. Ed. 2012, 51, 4431
Contact us
York Biomedical Research Institute
ybri@york.ac.uk
B/H/002, Department of Biology, Wentworth Way, University of York, York, YO10 5NG
Twitter
Contact us
York Biomedical Research Institute
ybri@york.ac.uk
B/H/002, Department of Biology, Wentworth Way, University of York, York, YO10 5NG
Twitter