Understanding how virus particles assemble
Lead researcher: Professor Fred Antson, Department of Chemistry
Viral infections caused by the family of herpes viruses, such as HSV1, Epstein-Barr, Cytomegalovirus and Varicella Zoster cause lifelong infections that are impossible to eradicate using current treatments. These viruses may remain dormant for years, hiding from the immune system, only to reactivate and cause illnesses later, often leading to serious health disorders including cancer.
During an active phase, new copies of the virus’ genes are made and loaded into capsids, as shown on figure 1 , to make new virus particles that then burst out of the host and spread to other cells.
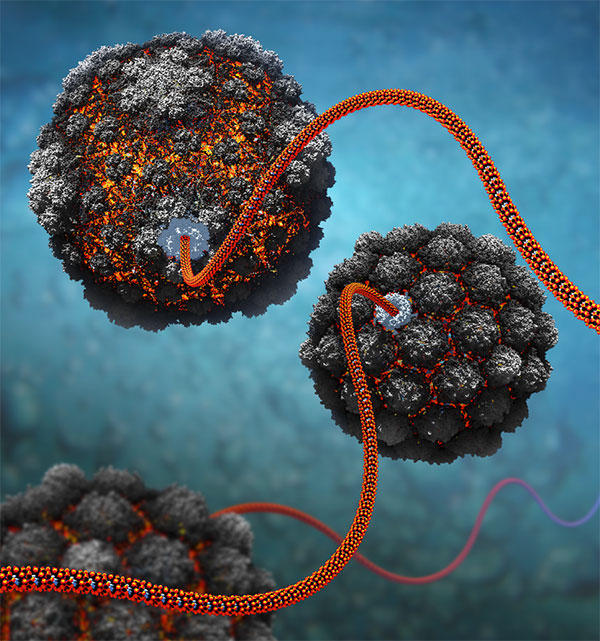
Figure 1
To understand the mechanism of virus assembly, the Antson group is using model systems derived from bacteriophages, close evolutionary relatives of herpes viruses. The main emphasis is to image molecular machines using cryo-electron microscopy and X-ray crystallography. The research provides detailed information about the structure of individual proteins, how they fit together and how they work [1,2].
In addition to the research on dsDNA viruses such as phage, Antson group is working on understanding how ssRNA viruses store and protect their genome. Using cryoEM, Antson group reconstructed 3D structure of the Nipah virus nucleocapsid assembly, Figure 2, showing how genomic RNA wraps around the nucleoprotein that provides protection and assists with replication. Structural details enable comparisons with other known viruses, and help assess which sections could be targeted by the immune system and potential therapies [3].
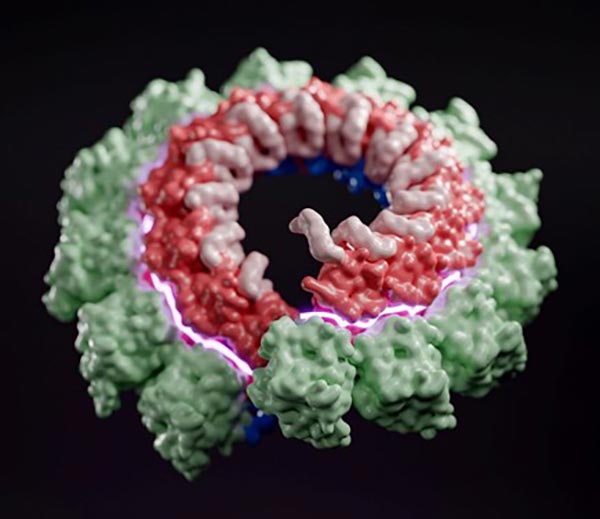
Figure 2
This research addresses the knowledge gap in the molecular interactions within protein-nucleic acid machines critical to viral replication and assembly. Knowledge of the architecture of these machines and the structure of their functional domains and active sites will help us understand the underlying molecular mechanisms. With such knowledge we can more effectively develop genetic and chemical intervention strategies against viral infections.
In the longer term, the research aims to translate structural and mechanistic findings into anti-viral therapies and nanomedical applications [4].
References
- Bayfield OW, Steven AC, Antson AA: Cryo-EM structure in situ reveals a molecular switch that safeguards virus against genome loss. eLife 2020; 9: e55517, DOI: 10.7554/eLife.55517.
- Bayfield OW, Klimuk E, Winkler DC, Hesketh EL, Chechik M, Cheng N, Dykeman EC, Minakhin L, Ranson NA, Severinov K, Steven AC, Antson AA: Cryo-EM structure and in vitro DNA packaging ofa thermophilic virus with supersized T=7 capsids. Proc. Natl. Acad. Sci. USA, 2019, 116:3556-3561.
- Ker D-S, Jenkins HT, Greive SJ, Antson AA: CryoEM structure of the Nipah virus nucleocapsid assembly. PLOS Pathogens, 2021, 17:e1009740.
- Cressiot B, Greive SJ, Si W, Mojtabavi M, Antson AA, Wanunu M: Thermostable Virus Portal Proteins as Reprogrammable Adapters for Solid-State Nanopore Sensors. Nature Communications, 2018, 9:4652.
Contact us
York Biomedical Research Institute
ybri@york.ac.uk
B/H/002, Department of Biology, Wentworth Way, University of York, York, YO10 5NG
Twitter
Contact us
York Biomedical Research Institute
ybri@york.ac.uk
B/H/002, Department of Biology, Wentworth Way, University of York, York, YO10 5NG
Twitter