Structure of heat-loving virus revealed
Cryo-electron microscopy has provided vital clues to the nature of virus assembly
Studies of a virus isolated from natural hot water source in Kamchatka, Russia, have provided clues about how the capsid protein shells of viruses assemble and expand. Bacterial viruses (also called bacteriophages) which infect the Thermus thermophilus bacterium are found in natural hot water sources. Due to their high stability and evolutionary links with human and animal viruses, these bacterial viruses are excellent model systems for understanding virus assembly.
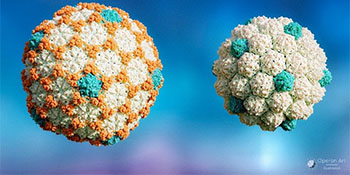
Expanded virus capsid (left), and the immature ‘procapsid’ prior to expansion (right).
In a study led by Professor Fred Antson published in Proceedings of the National Academy of Sciences, high resolution three-dimensional reconstructions of Thermus bacteriophage P23-45 revealed the structural changes that occur as the virus capsid shell matures and expands. These structures were obtained using advanced cryo-electron microscopy and data processing techniques, enabling atomic models to be built for the proteins in the capsid shell. The research unexpectedly showed that these capsids have evolved an increased storage capacity, without increasing the number of protein subunits that constitute the capsid shell.
Reflecting on the surprising discovery, Dr Oliver Bayfield said: ‘When we determined the capsid structures, we were surprised that they possessed what we call a triangulation number of 7, which relates to how many capsid proteins make up the capsid shell, despite the virus genome being double the size expected for this kind of capsid. We had to double-check the microscope was calibrated correctly, as it was quite unexpected’.
The virus utilises an unusual modification to a classical virus capsid protein, which allows a doubling of the capsid volume whilst maintaining its structural integrity up to the boiling point of water – as experienced in the hot spring environment. The researchers also developed a way to package DNA into the purified empty capsids in a test-tube, providing the opportunity to further study the mechanism of virus assembly under controlled laboratory conditions. In the future, these capsids also have significant potential to be employed as nanocages in biotechnological applications.
Professor Antson is part of York Structural Biology Laboratory which focuses on biological chemistry research, including structural and chemical biology to uncover the fundamental chemical bases for biological and biochemical processes. The research paper can be found here.