New enzymes synthesise amines with multiple chiral centres
Recent work performed in collaboration between the Universities of Manchester and York, with input from industrial partners, has uncovered a novel and powerful enzyme capable of synthesising amines with multiple chiral centres.
Chiral amines are ubiquitous in pharmaceuticals and agrochemicals, yet their preparation often relies on multi-step synthetic procedures which sometimes only proceed with difficulty. Furthermore, these valuable compounds must be manufactured asymmetrically, with control over each chiral centre, as biochemical properties can differ based on the chirality of the molecule. Each additional chiral centre in the molecule introduces significant complexity to this process.
Previously, this team of researchers led by Professor Nicholas Turner in Manchester have worked on imine reductase (IRED) enzymes capable of control over a single chiral centre during amine synthesis. However, in this new work, they have discovered an enzyme capable of also controlling two additional chiral centres.
The enzyme (EneIRED), identified from a panel of enzymes provided by Prozomix Ltd, can reductively couple a broad selection of α,β-unsaturated carbonyls with amines (see reaction scheme). In this way, not only is the chirality of the new amine group controlled, but also the chirality of the reduced alkene. This yields chiral amine products bearing three chiral centres (marked with asterisks in scheme).
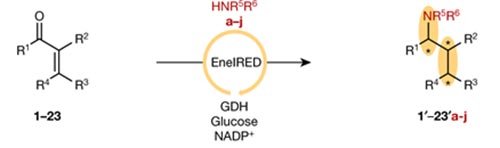
Reaction between α,β-unsaturated carbonyl compounds (left) and amines (red) catalysed by EneIRED to give the controlled synthesis of amines with three chiral centres.
Mechanistic and structural studies have been carried out to probe the catalytic process. Led by Professor Gideon Grogan in the Department of Chemistry, the team in York performed crystallographic and docking studies to understand the enzyme in molecular-level detail, giving insight into how the stereochemistry of both alkene and imine reduction could be governed by the enzyme active site. The predictions from the modelling mapped onto the experimentally observed outcomes for these reactions.
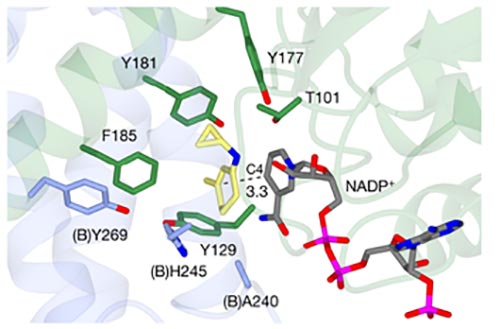
Active site of EneIRED complexed with co-factor NADP+ (grey). The X-ray structure has been used to model interactions with the reaction intermediate (yellow) in order to understand the chirality control of the reaction.
Reflecting on the work, Professor Grogan said: ‘These EneIRED enzymes possess broad scope, being able to convert many different precursors into valuable amines with multiple chiral centres in a one-pot, single-catalyst approach. We anticipate that further optimisation of the enzyme will enhance its practical utility, and look forward to working with our industrial partners, Prozomix and Pfizer, to develop industrial applications of this technology.’
This research has been published in Nature.