New Research Crosses the Solubility Rubicon
Recent research from the Department of Chemistry has reported a new synthetic approach to facilitate the modification of water-soluble biomolecules with water-insoluble carboxylic acids. Ultimately, this approach will help create a wide range of bioconjugates with therapeutic applications.
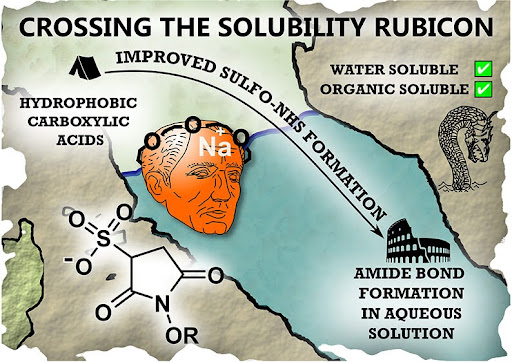
Amide bond formation between carboxylic acids and amines is a mainstay of medicinal chemistry: this is a critically important reaction in the synthesis of many pharmaceutical molecules. A major challenge in making new so-called “bioconjugate” therapeutics is how to couple together large biological molecules, which are soluble in water, and small chemical molecules which do not dissolve in water.
In their new publication, ‘Crossing the Solubility Rubicon: 15-Crown-5 Facilitates the Preparation of Water-Soluble Sulfo-NHS Esters in Organic Solvents’, researchers from York tackle this solvent-incompatibility problem. They apply a classic synthetic method using a crown ether to modify the solubility, and therefore reagent compatibility, of "activated esters" of carboxylic acids, hence solving this long standing bioconjugation problem in an elegant way.
Specifically, this work describes how it is possible to perform an esterification reaction between the highly polar Sulfo-NHS reagent, a mainstay of amide bond formation chemistry, and a non-polar carboxylic acid. Commercially available 15-crown-5 was used to coordinate the sodium cation of the Sulfo-NHS, creating a new reagent which readily reacts with non-polar carboxylic acids, generating a “crown-sulfo-ester” product which can be easily transferred into water for subsequent protein-labelling reactions. The idea of “crowning” the molecule inspired the paper title and artwork, with the Rubicon being the famous river crossed by Julius Caesar to enter Italy and become dictator of the Roman Empire.
The work was led by postdoctoral researcher, Dr Nicholas Yates, working in the group of Prof. Alison Parkin. Nick describes the intended impact and applicability of the work as: “By providing a simple, low-cost, and scalable method that allows water-soluble Sulfo-NHS-type esters to be produced conveniently and in high yields from water-insoluble carboxylic acids, we hope that amide-bond forming reactions using biomolecules will become less arduous for other researchers focused on the preparation of important therapeutics such as antibody-drug conjugates.”
The paper is available online: https://pubs.acs.org/doi/full/10.1021/acs.bioconjchem.3c00396