Professor Alison Parkin
01904 322561
Email: alison.parkin@york.ac.uk
Contact Alison for opportunities in the group
Research
Electrochemical insights into redox-active metalloproteins, bacterial metabolism and catalysis
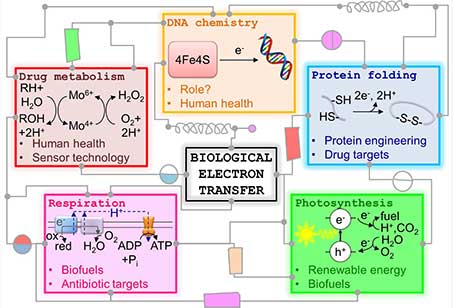
I am interested in how oxidation state changes at transition metal centres in proteins activate chemical reactions which are essential for life. Understanding such processes is important across a wide range of fields: a fundamental understanding of how bacteria derive the energy for life is essential if we are to develop new antibiotics to halt the rise in drug-resistant pathogens; learning how H2 and other small molecule activation occurs efficiently and rapidly at non-precious metal centres teaches us how to design better industrial catalysts; and finding out how novel proteins function in humans reveals how we work as big reaction vessels of living chemistry.
In the laboratory the main technique used to interrogate these redox reactions is film electrochemistry. This method permits precise control of the reduction-potential of the protein so that measurement of current provides a quantitative measure of the rate of redox reactions. Thermodynamic and kinetic reaction parameters are determined quantitatively, and enzymatic reaction mechanisms can be probed through analysis of substrate catalysis and inhibitor sensitivity. To complement the functional insight that electrochemistry provides, structural information is determined with molecular biology, protein crystallography, electron paramagnetic resonance and infra-red spectroscopy.
We also apply our electrochemical toolkit to probe synthetic CO2 chemical reactions, collaborating with Prof Mike North to develop new capture and utilisation methodologies and with Prof Peter O'Brien and Dr Victor Chechik to harness Kolbe chemistry for late-stage functionalisation.
Protein film Fourier transform alternating current voltammetry
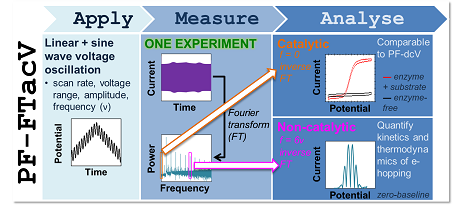
We are working with the world-class Australian electrochemist Professor Alan Bond to develop his technique, large amplitude Fourier transform alternating current voltammetry, as a powerful tool for studying redox active proteins and enzymes via film electrochemistry. We refer to this technique as “PF-FTacV” and our joint review paper explains why this technique yields such highly sensitive and powerful measurements:
H2 as a fuel in bacterial disease
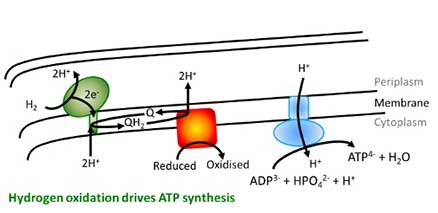
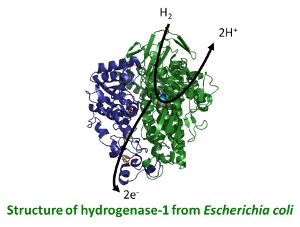
In living cells, oxidation and reduction reactions are coupled together across a membrane to generate electron gradients which ultimately provide the energy for ATP synthesis and life. Many disease causing bacteria are able to use H2 as a fuel in this process, with H2 uptake (H2 2H+ + 2e-) having proved very important in enabling Salmonella, Mycobacterium tuberculosis and Helicobacter pylori (a stomach cancer-causing agent) to survive. The function and structure of these enzymes and the biosynthetic pathways of assembly will be probed to understand how this chemistry works and how it can be shutdown in order to try and develop new antibiotic drugs. Research in this area is in collaboration with Professor Frank Sargent (University of Dundee) and Professor Greg Cook (University of Otago, New Zealand).
Technologically useful bio-catalysts
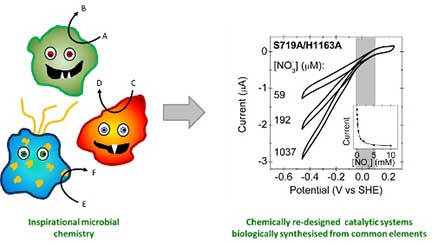
Microbes are fantastically inspirational chemical factories: they produce highly efficient and active catalysts which use common metals and often operate at standard temperature and pressure. Much of the small molecule chemistry which occurs in bacteria is industrially important to humans and studying the enzymes which catalyse this chemistry can teach us how the transition metal centres function. We can then manipulate the DNA of the bacteria in order to force the bacteria to biologically synthesis novel ligand environments to specifically re-tune the chemical specificity. Some of the enzymes being studied are:
- Acetylene hydratase catalyses the reaction HC≡CH + H2O → HC(O)-CH3. This is an important example of C-H bond activation, a class of reactions of fundamental importance to organic synthesis. This project is in collaboration with Dr John Slattery (York) and Professor Neil Bruce (York)
- Polysaccharide oxygenases are Cu-dependent enzymes important in the breakdown of chitin, cellulose and other recalcitrant polysaccharides which may play a critical step in the development of biofuel technologies. This project is in collaboration with Professor Paul Walton (York) and Professor Gideon Davies (York)
- Nitrate reductase catalyses NO3- + H2O + 2e- → NO2- and has applications in the development of galvanometric biosensors for water quality measurement. This project is in collaboration with Professor Paul Walton (York) and Professor Joel Weiner (University of Alberta, Canada).

