Reverse or Anatomical replacement for Painful Shoulder Osteoarthritis, Differences between Interventions (RAPSODI): a multi-centre, pragmatic, parallel group, superiority randomised controlled trial
Study Aim:
To find the best type of joint replacement for the treatment of painful osteoarthritis of the shoulder in adults aged 60 years or older.
Background:
With increasing age, shoulder osteoarthritis is common and causes severe pain and stiffness making everyday activities difficult. A shoulder replacement is an effective solution, reducing pain and allowing the shoulder to function better.
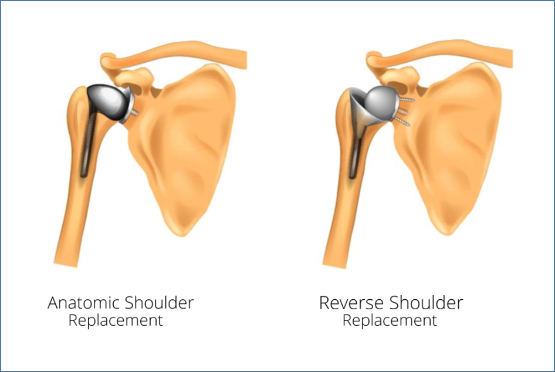
There are two types of shoulder replacement:
- Anatomic Total Shoulder Replacement which relies on the tendons (Rotator Cuff) around the shoulder to be intact and healthy.
- Reverse Total Shoulder Replacement, which is usually used when the rotator cuff becomes weaker or torn.
The rotator cuff can weaken with age which may cause an anatomic replacement to stop working. This could mean a further operation to change the shoulder to a reverse total shoulder replacement. For this reason, an increasing number of patients are offered reverse shoulder replacements even when their rotator cuff is intact. Currently, there is no scientific evidence to support this change and no guidance to recommend which is the best type of shoulder joint replacement. This trial aims to provide clinical evidence for the superiority (or not) of reverse shoulder replacements over anatomic. We also aim to inform which is the most cost-effective option to the NHS, explore patients’ expectations of the two types of shoulder replacement, and to link with the National Joint Registry for data on patient mortality and revision rates.
The Study:
The research will be undertaken in NHS shoulder arthroplasty units in England, Wales and Northern Ireland. Patients will be screened for eligibility (suitable for shoulder arthroplasty and intact rotator cuff) and invited for recruitment prior to surgery. Patients will be randomised prior to surgery and will be blinded to which type of replacement they received. We will recruit 430 patients and follow their recovery and outcomes periodically over the course of 24 months. We will also conduct a nested qualitative study of 20 participants who will be interviewed at 2 and 12 months after surgery.
The study Sponsor is Wrightington, Wigan & Leigh NHS Foundation Trust with funding from the National Institute for Health Research Health Technology Assessment Programme. Professors Ian Trail at Wrightington and Joe Dias at University Hospitals of Leicester NHS Trust are the joint Chief Investigators. The RAPSODI-UK trial group is collaborating with a linked application through the NIHR-NHMRC collaboration to run a parallel trial to the same protocol in Australia. Professors Richard Page at Deakin University, Australia and Nadine Foster at the University of Queensland, Australia are co-applicants of the RAPSODI-UK trial.
Recruitment Materials for Site Staff
RAPSODI - Recruitment Infographic 2025 (PDF ![]() , 2,977kb)
, 2,977kb)
RAPSODI 'PARABLE' Recruitment Animation for Sites
Principal Investigator (PI) and Associate PI Meeting on 18/10/2024
Topics Discussed:
Existing Literature (Ian Trail) [0:00 - 12:16]
Intact Rotator Cuff (John Geoghegan) [12:17 - 20:54]
Glenoid Erosion (Harvinder Singh) [20:55 - 32:01]
Trial Progress (Stephen Brealey) (including discussion about the age of the target population, advanced imaging practice, and private sector) [32:02 - 52:45]
(Questions/Discussions were held after each presentation)
Privacy Notice: How we use your research data
Funding
| Funders(s) | NIHR HTA Project: NIHR133418 |
| Start Date | March 2022 |
| End Date | April 2027 |
Members
YTU Team
- Stephen Brealey
- Hannah Connolly
- Caroline Fairhurst
- Catherine Hewitt
- Heather Leggett
- Catriona McDaid
- Fi Rose
- Fraser Wiggins
External Members
- Prof Ian Trail (co-CI) - Wrightington, Wigan & Leigh NHS Foundation Trust
- Prof Joseph Dias (co-CI) - University Hospitals of Leicester NHS Trust
- Associate Prof Harvinder Singh - University Hospitals of Leicester NHS Trust
- Prof Adam Watts - Wrightington, Wigan & Leigh NHS Foundation Trust
- Mrs Alison Robinson - Wrightington, Wigan & Leigh NHS Foundation Trust
- Mrs Katy Boland - Wrightington, Wigan & Leigh NHS Foundation Trust
- Mrs Lindsay Cunningham - Wrightington, Wigan & Leigh NHS Foundation Trust
- Mr Michael Walton - Wrightington, Wigan & Leigh NHS Foundation Trust
- Dr Elizabeth Camacho – The University of Manchester
- Dr Matthew Parkes - The University of Manchester
- Dr Rachael Powell - The University of Manchester
- Dr Chris Sutton - The University of Manchester
- Mr John Geoghegan - Nottingham University Hospitals NHS Trust
- Prof Nadine Foster – The University of Queensland
- Prof Richard Page - Deakin University
- Mrs Tracy Grey – Equality and Diversity Lead
- Mr William Greenwood – PPI representative
Blinding Guidance for Sites
For detailed information and guidance about blinding on RAPSODI please see this ‘Blinding Guidance for Sites’ document
Strength assessment data collection
Patient Resources
Patient Information Animation
Please take your time to watch this Patient Information animation. It’s written just for you, and it’s really important because it explains everything you need to know about the study—what it involves, what to expect, and what your rights are. This will help you understand what you are agreeing to.
We want you to feel completely comfortable and informed before making any decisions. If anything is unclear or you have questions, the staff at the hospital will talk it through with you.