50-Year-Old Synthetic Challenge Solved
Posted on Thursday 6 January 2022
Phenols are compounds with high value in the pharmaceutical, agrochemical and polymer industries. In general, their synthesis relies on the conversion of an aromatic halide (C-X) bond into a phenol (C-OH) using a hydroxide anion reagent. However, this approach only works for fluorides and chlorides that are highly activated, and fails for bromides, iodides and non-activated systems.
With the goal of solving this problem, Dr Michael James decided to make use of a radical substitution approach. However, this has previously been considered impossible because hydroxide anions will not participate in this type of process.
Dr James and his research team of Masters students therefore developed a new reagent capable of facilitating this process and in collaboration with Professor Victor Chechik also developed a detailed mechanistic understanding of the way it works.
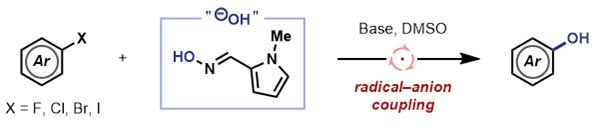
The oxime reagent (see blue box) is capable of transferring its hydroxyl group to an aryl radical (created on loss of the halide), enabling the smooth conversion of aromatic halides into phenols. The team were delighted to discover that this reagent was compatible with all aromatic halides, including the previously problematic bromides and iodides, and also worked effectively for non-activated systems.
The team hope that this ‘transition-metal free’ reagent will soon be commercialised and made widely available to others who wish to perform this kind of reaction. Dr James said “The conversion of a halide into a phenol looks such a simple process on paper, but for many years has been challenging except in very specific cases. This new carefully-designed reagent opens the possibility of a general transition-metal-free approach to this type of reaction which could be of high value in both academic and industrial settings.”
This research has been published open access in Chemical Science, the flagship journal of the Royal Society of Chemistry.
Dr James is funded by a Leverhulme Trust early career fellowship and this is his first fully independent research paper.
