CARE-ful Synthesis of Cyclic Compounds
A new synthetic method developed in York, conjugate addition/ring expansion (CARE), enables the simple synthesis of a wide range of cyclic compounds that can incorporate many different functional groups.
Compounds containing medium-sized rings have biological relevance and potential applications in the development of new drugs. Dr Will Unsworth and his research team have great interest in the development of new and improved synthetic routes to make this type of compound. With this synthetic target in mind, they combined two different reactions – conjugate addition and ring expansion (CARE) – into a single step.
Cascade reactions, which combine multiple reaction steps into a single operation are highly desirable in organic synthesis. They bring benefits in terms of making synthesis quick and easy, and avoiding the need to handle or isolate potentially toxic intermediates. In this work, Kleopas Palate, a PhD student in Dr Unsworth’s lab, combined conjugate addition and ring expansion into a single reaction step.
As shown in the Figure, this enables medium-sized ring and macrocyclic bis-lactams to be prepared from cyclic imides (blue), a,b-unsaturated carbonyls (red), and primary amines (green). The reactions are simple to perform, generally high yielding, and very broad in scope, especially with respect to the primary amine component. By varying the components used in the reaction it is possible to ‘program in’ a wide range of different functionalities – indeed a library of around 50 different cyclic compounds was prepared using this approach.
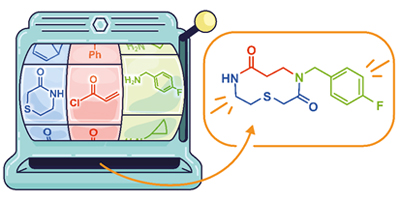
Image credit: Claudia Flandoli
Talking about the research Dr Unsworth said: ‘Our research team have been working on the development of ring expansion reactions for several years, but our previous methods have tended to be quite time-consuming. With the discovery of CARE, we can make the molecules we’re interested in much more quickly and easily, using a ‘greener’, more environmentally sound approach. This should dramatically increase the attractiveness of our synthetic methods for macrocycles in various applications – most notably the discovery of new pharmaceuticals and agrochemicals.’
This work is published in RSC Chemical Biology.