Clean oxidation reactions for use in the pharmaceutical industry
Working with a team from AstraZeneca, academics from the Departments of Chemistry and Biology have developed clean and effective enzyme-mediated synthetic methods for use in the pharmaceutical industry.
Oxidation reactions are key processes in synthetic chemistry and of vital importance in the pharmaceutical industry. However, mild and selective transformations often remain elusive. In particular, the oxidation of relatively unreactive C-H bonds often employs transition metal catalysts, an approach which can suffer from poor selectivity and low sustainability.
With this in mind, researchers have turned to using enzymes to try and catalyse such processes. In recent years, certain enzymes have risen to prominence, one such example being unspecific peroxygenase (UPO) enzymes. However, these enzymes require a precisely controlled supply of hydrogen peroxide – enough for the enzyme to work, but not too much to cause its degradation. Practically, this can be difficult to achieve.
In recent research, Professor Gideon Grogan and Dr Jared Cartwright, working together with a team of scientists from AstraZeneca, led by Dr Martin Hayes have developed an innovative solution to this problem. They have used a second enzyme, oxalate oxidase, to provide an in situ source of hydrogen peroxide that can then be utilised by UPO (see Figure).
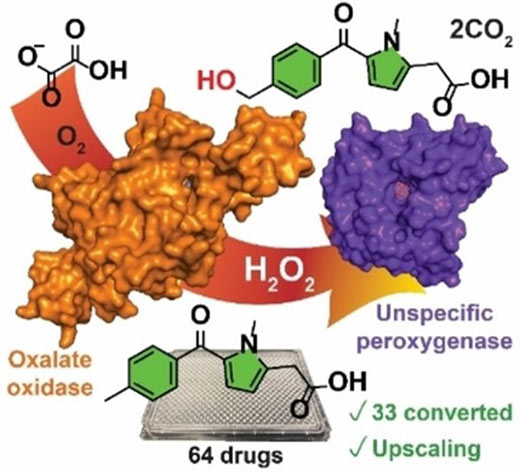
Oxalate oxidase is a well-known enzyme which produces hydrogen peroxide but has not been used before in combination with a UPO to drive synthetic reactions. In this work, the team demonstrated that this approach works for the oxidation of a diverse range of 33 different drug molecules. They also scaled-up one of the reactions to demonstrate proof of concept.
"Innovations like this provide us with the tools and technologies to make drug synthesis more environmentally friendly. This two enzyme in-one-pot approach ensures optimal hydrogen peroxide levels, enabling rapid access to industrially relevant molecules in a safe and sustainable way." says Dr Hayes.
Professor Grogan explains: "In the human body, pharmaceuticals are very often oxidised by metabolic processes. It is therefore vital for pharmaceutical chemists to test and understand the properties of oxidised drug molecules.
"This new approach provides easy access to such molecules, giving valuable information to our industrial partner, AstraZeneca. We hope that in the future, oxalate oxidase will go on to find a range of uses in synthetic chemistry as a simple and controlled way of generating the hydrogen peroxide required by other enzymes."
This research has been published in Angewandte Chemie Int. Ed.