Towards clinical coagulation control
The latest research from the Smith group presents heparin binding agents with greater stability and lower toxicity, which may be useful in helping control blood clotting after major surgery.
Heparin is a drug used during major surgery for blood thinning, however, once surgery is complete, the heparin must be removed so that clotting and healing can begin. This is currently achieved using protamine, which binds to heparin and removes it from the bloodstream. The protamine protein, which is extracted from shellfish, can have adverse effects and must be used with some caution. There is, therefore, considerable interest in creating synthetic versions of protamine that can do the same job.
Professor Smith and his research team have been interested in this problem since he learned about the issue from doctors when his husband Sam had a lung transplant in 2011.
Current approaches to synthetic heparin binders use small molecules of large polymers. Such small molecules can suffer from low binding affinity, given that heparin is itself very large. Conversely, although polymers bind heparin very well, their use can result in unwanted persistence in the body and toxicity effects.
The Smith group’s approach is to use small molecules that assemble together into a larger structure. In this way, they harness the advantages of strong binding from a larger structure, but with the potential for it to be broken down into smaller molecular parts with lower toxicity and persistence. They refer to their approach as self-assembled multivalency (SAMul), named after Dave’s husband, Sam.
In previous work, researchers in Smith’s lab demonstrated that this approach could be highly effective. The first generation systems they assembled, however, were not stable enough in highly competitive human blood serum, and started to break apart, lowering their binding affinity. This also gave them unacceptable toxicity profiles as they inserted into cell membranes.
In their new research, Smith and his team, along with Prof Sabrina Pricl from Italy and Dr Nadezda Apostolova from Spain, have demonstrated that by changing the ‘molecular glue’ that causes their SAMul systems to assemble, they can improve their stability. Computational work proved that it was much harder to disrupt their new system than their previous one. This enhances heparin binding in human blood serum. In addition, it lowers the toxicity, as the more stable systems do not disrupt cell membranes.
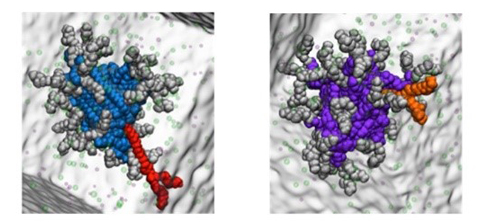
SAMul heparin binding systems. The old system (in blue) can be much more easily disrupted than the newly reported system (in purple) as proved by testing removal of the red subunit. This improves the heparin binding and lowers the toxicity of the new system.
With high affinity and acceptable toxicity profiles the new systems have considerable potential. Professor Smith says:
“We were delighted to see that by careful chemical programming of our SAMul systems, we were able to improve their performance against key clinical parameters. Future work in our labs in this area will focus on the translation of systems such as these into a real-world clinical setting. Our ultimate goal is to put new tools into the hands of teams responsible for surgery, making it safer and more predictable”.
The research is published in Biomaterials Science.