A multidimensional approach to blood serum analysis
A new spectroscopic technique that suppresses water absorptions allows infrared spectroscopic analysis of blood serum proteins for biomedical applications.
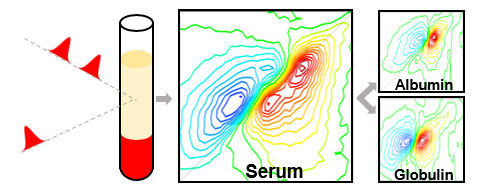
Infrared (IR) spectroscopy has long been a powerful tool used by chemists for determining the structure of molecules. IR produces a specific fingerprint for each molecule making it a potentially attractive method for analysing biofluids such as blood serum, where it could provide a broad chemical picture of the body’s metabolism. This would be a useful clinical aid for the early diagnosis of disease.
Until now biomedical applications of IR spectroscopy have been prevented because water absorbs IR light at the same wavelength as proteins, masking a vital piece of the blood serum molecular jigsaw.
Research by Samantha Hume, a PhD student in the research group of Professor Neil Hunt at the University of York and the group of Matthew Baker at the University of Strathclyde in collaboration with the Science and Technology Facilities Council (STFC) Central Laser Facility located at the Rutherford Appleton laboratory has now shown that 2D-infrared laser spectroscopy (2D-IR) can avoid this problem.
2D-IR suppresses the water signal relative to that of proteins, and also offers the added advantage that each protein produces a unique 2D signature that enables differentiation of proteins in complex mixtures in a way that standard IR methods simply cannot match.
The team demonstrated the approach by measuring the concentration ratio of albumin and globulins, the major protein fractions in serum across a clinically-relevant range. Each sample required about 1 minute to capture, with no need for pre-processing of the serum samples. It was also shown that 2D-IR spectroscopy can go beyond the albumin to globulin ratio by detecting levels of minor globulin proteins.
Professor Hunt said: “This new technique opens up a straightforward spectroscopic approach to measuring levels of serum proteins that are currently only accessible via biomedical laboratory testing and could play an important role in the analysis of complex samples.”
The research is published in Chemical Science, the flagship journal of the Royal Society of Chemistry.