New enzyme discovered in bacteria makes unusual sugar linkages
Posted on Friday 1 March 2024
Human cells are covered in sticky layer of sugars called sialic acids which can act as a barcode to be read by the immune system. This helps the body to differentiate between human cells and pathogenic invaders. However, some pathogenic bacteria are able to mimic these sugars in order to trick the immune system. One such sugar is pseudaminic acid, an
‘evil twin’ of human sialic acid, present on the surface of a number of multidrug resistant pathogenic bacteria including Acinetobacter baumannii, which causes hospital acquired infections around the world.
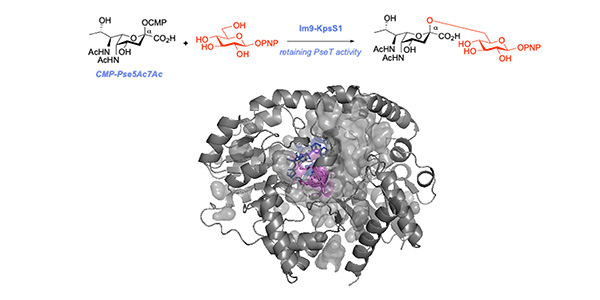
Although the structures of the sticky coat on the surface of the bacteria were already known to include a pseudaminic acid sugar, how this sticky coat was biosynthetically assembled by the bug was unknown. Research led by PhD student Abby Walklett in the lab of Dr Martin Fascione from the Department of Chemistry, in collaboration with Prof Gavin Thomas from the Department of Biology, has now shown that this pseudaminic acid sugar is installed using a previously unreported class of glycosyltransferase enzymes, that are specific for pseudaminic acid and build an unusual linkage through a novel enzymatic mechanism.
Dr Fascione said: “Pseudaminic acid is a non-mammalian sugar attached to the surface of a number of multi-drug resistant ESKAPE pathogens. However, its challenging structure has so far limited study of it as an antimicrobial target. In this work, we used our expertise in the chemical and enzymatic synthesis of the sugar to characterise a novel glycosyltransferase (GT) enzyme in a pathogenic Acinetobacter baumannii strain. This enzyme is the first reported GT to use pseudaminic acid and also proceeds with a retention of stereochemistry, a rarely reported GT mechanism- making it the prototypical member of a previously unreported class of enzymes.”
“The bacteria likely use these sugars to camouflage themselves from the immune system, so an increased understanding of this enzyme class and the mechanism they use will allow us to start designing chemical tools and inhibitors which could pave the way to new antibiotics for these pathogens”
This research has been published in Angewandte Chemie.
