International collaboration wins Royal Society of Chemistry Prize
An international research team, led by groups at The Crick (UK) and Stanford University (USA) and joined by Dr Jon Agirre (York Structural Biology Laboratory, Department of Chemistry), has been recognised with one of the inaugural Royal Society of Chemistry Horizon Prizes.
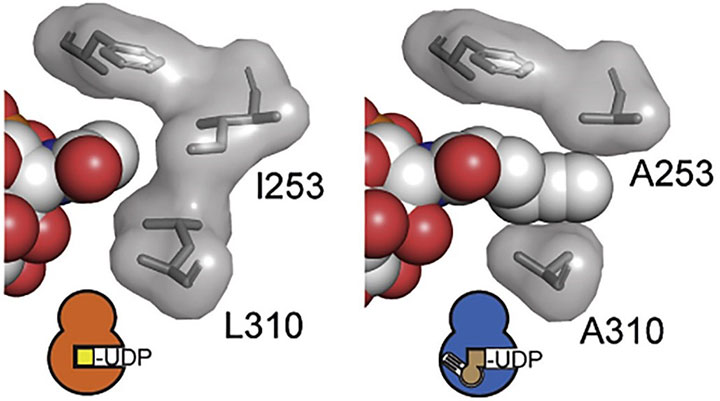
The prize recognizes the work of a team of more than 50 scientists from many different backgrounds, highlighting the importance of collaboration and diversity in science. Their careful work developed precision chemical tools to identify substrates of a glycosyltransferase enzyme directly in living cells.
The multidisciplinary team, led by Dr Ben Schumann (The Crick, UK) and Professor Carolyn Bertozzi (Stanford University, USA), used the ‘bump-and-hole approach’ to work with a modified version of a biologically-important enzyme that plays a key role in adding sugars to proteins. The modified poly-peptide N-acetylgalactosaminyl transferase (GalNAc-T) was capable of accepting tagged substrates that can be tracked in living cells. With these new tools, the scientists managed to pinpoint the ‘landing area’ of the modified substrates with unprecedented accuracy, hence identifying which proteins can receive a sugar substrate from GalNAc-T.
The work includes, among many other techniques, a wealth of protein crystallography. Dr Jon Agirre (YSBL, Department of Chemistry), who currently holds the Royal Society Olga Kennard Research Fellowship – a special position created for a Royal Society Research Fellow working in crystallography, and supported by a generous donation from the Cambridge Crystallographic Data Centre – assisted the team with the refinement of the atomic structures of the complexes. Dr Agirre is an expert in the refinement of carbohydrate structures, and ensured that the resulting atomic structures made chemical sense.
Dr Agirre explained: "This is the first time a glycosyltransferase's activity has been probed with the ‘bump-and-hole’ tactic and the results could not be more exciting. My participation in this study has allowed me to put our computational procedures to the test with challenging data. I am incredibly grateful to Ben and Carolyn for letting me take part in this and delighted to see this international collaboration recognised with an RSC Award."