 Download this page as an activity sheet (pdf, 70KB). Download this page as an activity sheet (pdf, 70KB).
Supermolecules
In this activity, you can:
Supermolecules and your washing up
Washing up liquid contains detergent molecules. These molecules have a very
specific structure – with a polar head group and a long hydrophobic (water-hating) tail. When you dissolve the detergent in water, it assembles automatically to form a supermolecule. The hydrophobic tails interact with one another and want to hide from the polar water molecules. The charged head group, in contrast, wants to interact with the polar water molecules. These interactions cause the detergent molecules to assemble into a spherical supermolecule called a micelle, like the one in our diagram:
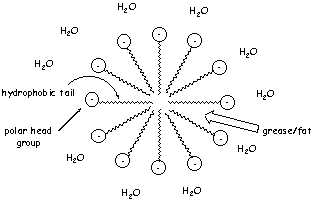
It is these giant micelles that make the washing up liquid work. Any oily,
greasy substances on your plate will want to go inside the micelle. Inside the micelle, the greasy substances can avoid the polar water molecules and interact instead with the long hydrophobic
tails of the detergent. Using these supermolecules, therefore, your plates will end up sparkling clean, as the micelles encapsulate all the greasy waste. But don't forget, detergents are not just for washing up and you can find a more frivolous use for detergents here – blowing bubbles!
You don't only wash up with supermolecules – sometimes you even eat them!
Many foods contain both oil and water and somehow they have to mix in order to give a smooth tasty product. In order to achieve this, molecules called emulsifiers are added. These emulsifier molecules form supermolecules, rather like the micelles in washing up liquid, allowing the oil and water to mix.
Salad cream is a good example of a product containing emulsifiers. Egg yolk can be used as a natural emulsifier.
Further investigations
How many different foods can you find in your kitchen that contain emulsifiers
(look on the lists of ingredients)?
What do the different foods have in common?
Back to the top.
Build your own supermolecules
To make your own supermolecules, you'll need:
- a small clear plastic bottle (a small soft drink bottle is ideal),
- a measuring jug,
- some cooking oil (olive oil or sesame oil works well),
- washing up liquid.
To build your supermolecules:
- Remove any labels from the bottle so you can see inside it clearly.
- Using a measuring jug, measure 250 ml of tap water into the bottle.
- Then add enough oil to form a thin layer on top of the water. This will be about 20 ml of oil (or 2 dessertspoonfuls). It is best if the oil is coloured, such as olive oil or sesame oil.
- Shake the bottle and leave to settle. You should clearly see the oil floating in a layer on top of the water.
- Add a small amount of washing up liquid to the bottle. Shake well. What do you observe? There will be lots of bubbles and the oil should begin to mix with the water, giving a cloudy solution. This is because the oil is going inside the micelle supermolecules you've made, which mix with the water.
Further investigations
How much washing up liquid do you need to get reasonable mixing of the oil and water?
Do different brands of washing up liquid have different efficiencies?
Back to the top.
Blue sky research!*
It's an age-old question, but just why is the sky blue? To investigate, why not
create some blue sky in your kitchen? You'll need:
- a clear plastic or glass container, ideally square/rectangular & straight sided,
- a powerful torch to play the part of the sun,
- a small quantity of milk or powdered milk.
To create your blue sky:
- Fill the container with tap water, then add a very small quantity of milk powder or
a drop of liquid milk.
- Look at the container from the side and gradually add a bit more milk until you notice a bluish tinge reminiscent of the sky. Remember, you can always add a bit more milk but you can't take any out! Stir well.
- Shine the torch through and look at the container from the side, at right angles to the
direction of the light beam. Then look directly into the light beam from the opposite
side of the container. It might help to close the curtains in the room before you do this.
- In each case, what do you notice? You should see a blue sky looking at the container from the side; looking directly through the water at the light source causes it to appear yellow or red depending upon the amount of milk used.
- Try adding a little bit more milk/powder and see what effect it has.
How does this work? – the science bit
Milk is an emulsion of fat droplets in water, stabilised by protein molecules acting
as emulsifiers and forming supermolecules. These droplets scatter light; in the same way, molecules in the atmosphere scatter light giving the appearance of a blue sky.
White light from the sun, or the torch, contains all the colours of the rainbow. Certain colours are scattered more than others – you can see this as you view the container from different angles. Which colour is scattered the most and which the least?
Can you make a container sunset? – further investigations
It's even possible to set up and watch your very own sunset in a tank! A few extra
chemicals are required so why not read about Tyndall's experiment? You can find out more about Tyndall's experiment here.
*By the way, blue sky research is speculative work that isn't expected to produce immediate benefits, but may one day lead to a scientific breakthrough. That day could be many decades away! | 
