The EXTEND trial: EXTENDed antibiotics to improve outcomes in patients with complicated intra-abdominal infection
What is the EXTEND Trial?
What are complicated intra-abdominal infections?
Bacteria live in the intestine to help digest food. If the intestine is damaged, for example by an operation or a disease such as cancer, bacteria can leak into the space surrounding the intestine (called the abdominal cavity) and cause serious infections known as complicated intra-abdominal infections. Over thirty thousand patients per year suffer from this type of infection.
Why is research into complicated intra-abdominal infections needed?
The care of patients with complicated intra-abdominal infections is a big concern for doctors. The damaged area of the intestine may need to be removed and antibiotics are used to kill any bacteria left in the abdominal cavity. However, this treatment does not seem to be working as well as hoped. In up to half of patients the original infection comes back or patients develop another infection. This means that these patients may need a second round of treatment, including antibiotics and/or an operation.
Research from other trials has suggested that longer courses of antibiotics may offer benefits for patients with serious abdominal infections. If longer courses of antibiotics are better at curing and preventing infections, they may be better at keeping patients out of hospital. This may reduce the chance patients will catch antibiotic resistant infections.
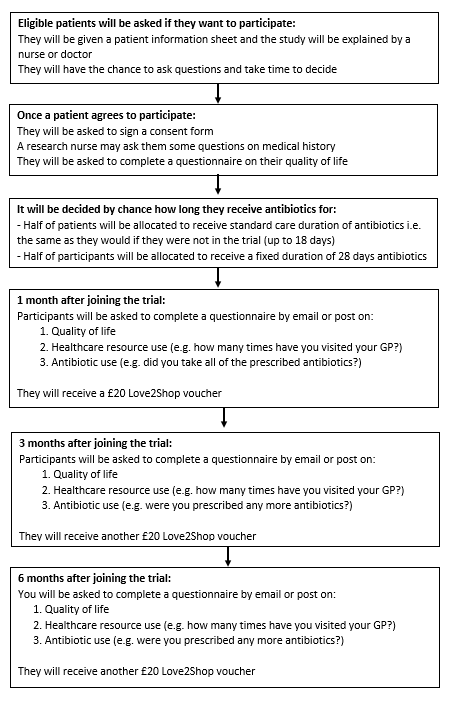
What will the EXTEND trial involve?
To out whether longer antibiotic courses are better for patients with complicated intra-abdominal infections, the EXTEND trial will group patients by chance into one of two treatment groups. One group will take antibiotics as normal, which is decided by their own doctor (often about 7 to 18 days). The other group will take antibiotics for four weeks.
We will monitor patients in both groups over six months to see whether the treatments prevent the return of the original infection and the development of new infections. Patient quality of life will be assessed during this time through the completion of a questionnaire which asks whether patients have any problems with mobility, self-care, their usual activities, pain/discomfort and anxiety/depression.
What impact will this have?
The results of the research will be shared throughout the NHS and charities to help doctors decide if longer courses of antibiotics will benefit patients with complicated intra-abdominal infections.
The study is now open to recruitment: please see the information below for patients or research staff.
EXTEND TRIAL: Map of participating sites
Conflict of Interest
All individuals involved in the management and design of the EXTEND trial (see “Members" tab) are employed by the NHS and/or a university. There are no conflicts of interest to disclose. This study aims to benefit patients.
Approvals
The EXTEND trial has received approval from the HRA and Leeds West Research
Ethics Committee (IRAS Reference: 302989; REC Reference: 22/YH/0023).
Privacy Notice: How we use your research data
For Patients
For Research Staff
The Associate PI Scheme
Complicated Intra-Abdominal Infection (cIAI): Study Day/EXTEND Trial Investigators Meeting 15th March 2023
Funding
| Funders(s): |
NIHR Health Technology Assessment (ref 131784) |
|---|---|
| Start Date: | December 2021 |
| End Date: | May 2026 |