Protein Allostery
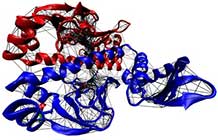
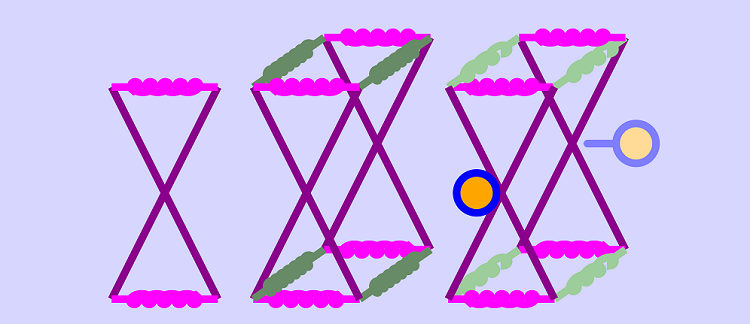
Wodak, S. J. et al., ‘Allostery in Its Many Disguises: From Theory to Applications’, Structure, 27, 566-578 (2019)In this theme of our research we explore in detail three fundamental ways in which randomness and noise are recruited in biology. The first example is right at the heart of information-processing in cells. 'Allostery' is the effect by which a protein molecule binds to another molecule (either a smaller species, or a giant molecule like DNA) if and only if a second 'signalling' molecule is also bound to it, at a different site. The presence of the signalling molecule is felt 'at a distance' at the other binding site. We are developing theoretical and computational tools to explore how the background of thermal fluctuations can be used to carry the signal, and learn from biology about the physics of fluctuating elastic matter.
I. Dubanevics, I and T.C.B. McLeish, 'Computational analysis of dynamic allostery and control in the SARS-CoV-2 main protease', Interface, 18, 20200591 (2021)
Schaefer, C., von der Heydt, A. C., McLeish, Tom, ‘The ‘allosteron’ model for entropic allostery of self-assembly’, Philosophical Transactions, 373, 20170186 (2018)
T.L. Rogers et al., “Modulation of Global Low-Frequency Motions Underlies Allosteric Regulation: Demonstration in CRP/FNR Family Transcription Factors”, PLOS-Biology, 11(9): e1001651 (2013)
