PROximal Fracture of the Humerus: Evaluation by Randomisation Trial no. 2 (PROFHER-2 Trial)
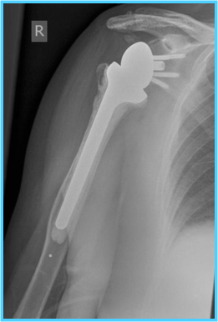
Fractures of the proximal humerus are common in people over 65 years of age who have had a fall. When the bone is broken into more than two parts it is considered complex and there are three treatments that are regularly used: hemiarthroplasty, reverse shoulder arthroplasty, and non-surgical care. Hemiarthroplasty involves replacing only the broken 'ball' of the joint and reverse shoulder arthroplasty replaces both the ball and socket, but replaces the ball with a socket and the socket with a ball (hence ‘reverse’). Non-surgical care is where the arm is supported in a sling to allow the broken bone to heal naturally. It is not known which of these is the most effective treatment.
PROFHER-2 will assess whether reverse shoulder arthroplasty is more effective than hemiarthroplasty for complex fractures of the proximal humerus and if these methods are more effective than non-surgical treatment. This will be a multi-centre randomised controlled trial where patients will receive one of these treatments and will also receive physiotherapy. The study aims to randomise 380 patients, 152 to each of the surgical treatments and 76 to the non-surgical treatment. The primary outcome that will be measured is change in the Oxford Shoulder Score after 24 months. The associated costs of these treatments to the NHS will also be evaluated.
The PROFHER-2 Trial continues to include new participants and follow-up existing participants during the COVID-19 pandemic. The NHS hospitals involved have been assessed to ensure they have adequate safety measures in place. Study procedures have been adapted to limit any potential risks to participants due to COVID-19. This includes remote follow-up options for new recruits and existing participants. Please get in touch with the study team using the emails addresses under “contact us” below for further information. If you are an existing participant, please contact your relevant hospital team in the first instance. This contact should be on your Patient Information Sheet.
Privacy Notice: How we use your research data
Funding
| Funder(s) | NIHR HTA |
| Start Date | 1 December 2017 |
| End Date | 31 November 2023 |
Contact Us
- If you are interested in becoming a participant in the trial, Claire Whitmore: claire.whitmore@york.ac.uk or tel: 01904 3216407
- If you are interested in becoming a trial site (or are an existing one), Ms. Catherine Arundel: catherine.arundel@york.ac.uk
- If you have a media or scientific enquiry, Dr. Puvan Tharmanathan: puvan.nathan@york.ac.uk