Parahydrogen
Scientists in CHyM use one of the simplest molecules to solve sensitivity problems in scientific and clinical measurements, parahydrogen.
What is parahydrogen?
Parahydrogen is a key component of the research conducted in CHyM. It is one of the chemical feedstocks which is used to increase the sensitivity of NMR and MRI measurements.
Parahydrogen is one of two magnetic forms of hydrogen gas (H2), the other is orthohydrogen. At room temperature a volume of hydrogen gas contains 75% orthohydrogen and 25% parahydrogen.
The difference between para and orthohydrogen is the spin of the hydrogen nuclei. In orthohydrogen the spins of the two hydrogen nuclei are aligned, in parahydrogen, they are opposed. This effect results in a tiny energy difference between the two magnetic states.
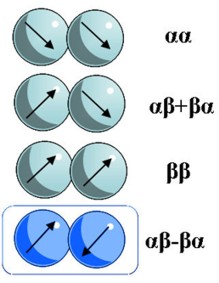
This figure shows the four spin combinations of dihydrogen split into two isomers of orthohydrogen and parahydrogen. The highlighted box indicates lowest energy parahydrogen state that isused in CHyM’s studies.
How do you make parahydrogen?
Making parahydrogen is relatively simple: higher-energy orthohydrogen is made into parahydrogen using low temperatures and a suitable catalyst.
Parahydrogen is the lowest energy form of H2, so by cooling H2 down (in the presence of a catalyst), more molecules enter this state.
The lower the temperature the more parahydrogen is present. At room temperature H2 contains 25% parahydrogen. By cooling the hydrogen down to -196 °C using liquid nitrogen, the proportion of parahydrogen is increased to around 50%. Further cooling to -250 °C (20 K) results in >99% parahydrogen.
| Temperature | % parahydrogen |
|---|---|
| 25 °C | 25 |
| 0 °C | 25.13 |
| -120 °C | 28.54 |
| -198 °C | 51.86 |
| -250 °C | 99.82 |
| -273 °C | 100 |
In addition to cold temperatures a catalyst is needed to allow the conversion of orthohydrogen to parahydrogen. The catalyst can be charcoal or iron oxide (or another ferromagnetic material) which allows the rapid conversion to parahydrogen to take place.
Once created the parahydrogen can be removed from the presence of the catalyst and warmed to room temperature, keeping the high levels of purity obtained in the process.
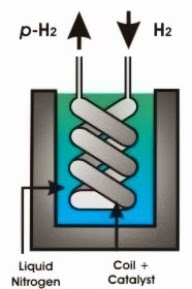
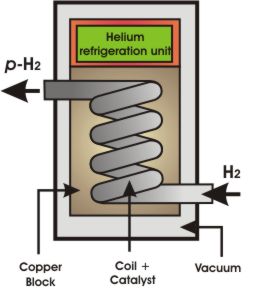
The following diagrams show methods for producing parahydrogen. The first diagram shows how to achieve 50% parahydrogen by passing H2 gas through a catalyst-containing coil at a temperature of -196 °C. This temperature is achieved by immersing the coil in liquid nitrogen. The second diagram shows how to produce parahydrogen at >99% purity using a helium refrigeration unit to achieve temperatures of -250 °C.
How can you use parahydrogen?
Once created, parahydrogen can be used as a source of polarisation for scientific and clinical measurements. By transferring the polarisation into other materials using hyperpolarisation, chemical and biological processes which were previously invisible can be detected using NMR and MRI measurements.
General enquiries
- Centre for Hyperpolarisation in MR (CHyM)
Tel: 01904 328886
Further information