 Download this page as an activity sheet (pdf, 99KB). Download this page as an activity sheet (pdf, 99KB).
Red Hot, Cool Chemistry
In this activity, you can:
Please follow any safety instructions highlighted like this in red.
Using chemistry to make chocolate ice cream

"What a salesman!"
This must be one of the best uses of science in the kitchen! You will need:
- 1 tablespoon of cream,
- 2 tablespoons of milk,
- 1 tablespoon of chocolate powder,
- ice cubes,
- salt,
- two bowls: one large, one small.
Place the cream, milk and chocolate powder into the smaller bowl. Put a bed of ice
cubes into the larger bowl and sprinkle with salt. Press the smaller bowl into the
salted ice. Add another layer or two of ice and salt around the smaller bowl, then cover with a tea towel
and leave it to sit. Stir the ice cream mixture every few minutes.
What's going on? – the science bit
Well, we all know that water freezes at 0 °C, but when we add an impurity to the
water, such as salt, we can lower the temperature at which ice melts. Why not experiment by measuring the temperature of the ice/water whilst making your ice cream? This same principle is used when we put salt on the roads in winter to make them safer to drive on.
Can you make water unfreezable? – further investigations
So, we know that adding salt stops water from freezing at the usual 0°C.
We can investigate this by adding some salt to water and putting it in the
freezer. Using a thermometer, find how cold the water will get before it freezes.
Does adding more salt allow it to go even colder without freezing? (you may need to
turn the freezer down, but make sure you ask permission!) How low can you go?
Back to the top.
Eggciting chemistry
Everybody knows that when you boil an egg it goes hard, but do you know why? At what temperature does it happen?
Here's a simple experiment to find out at what temperature the egg changes. All you'll need is a kitchen and a thermometer. Separate the white of an egg and add it to a small pan of water on the stove. Heat gently, using the thermometer to find out at what temperature the egg white goes opaque (clue: it's about 60°C). How accurately can you measure this?
Egg white is made of proteins: big, complicated molecules built into a very precise shape, like a dinosaur skeleton. Heating the egg causes the protein to shake and eventually collapse. The temperature at which the egg goes hard is the temperature at which these proteins collapse.
Back to the top.
How to make your own thermometer
How do we know how hot something is? An easy way is just to test with your hand;
but what happens when it gets too hot or cold to touch safely? For a scientist, being able to
measure temperature accurately is very important. Using some simple science we can
make a thermometer! You will need:
- a straw,
- some modelling clay or Blu Tack,
- a small clear plastic bottle with lid,
- some food colouring.
Here's what you need to do:
- Pour cold water into the bottle until its about 1/4 full. Add a few drops of food
colouring.
- Make a hole near the top of the bottle and insert a straw so that it dips in the
water; make a tight seal around the straw using Blu Tack or modelling clay.
- Blow into the straw and make bubbles until the water rises halfway up.
- With a marker pen, mark the level of the water in the straw; this is room
temperature.
Experiment putting your thermometer in hot and cold places! The thermometer works
because as the temperature rises, the air inside the bottle expands and pushes the water up the straw. At cooler temperatures, the air in the bottle contracts and the water drops.
Back to the top.
Mpemba and how hot and cold water freeze
Mpemba was a young African boy who worked in Tanzania making ice cream. He was
making the ice he needed one day when he noticed that hot water freezes faster than cold water! None of the expert scientists would believe him but luckily one grown up, his science teacher, did. Together they managed to convince the experts that Mpemba was right. Not only that, but the experts named it The Mpemba Effect after him. To this day, no-one really knows why it happens.
You can try this in your kitchen – just take some hot water from the kettle and
some cold water and put them both in the freezer. Keep checking them. Which one freezes first? Does the size and shape of the container have an effect? Does the type of container used affect which freezes first?
Back to the top.
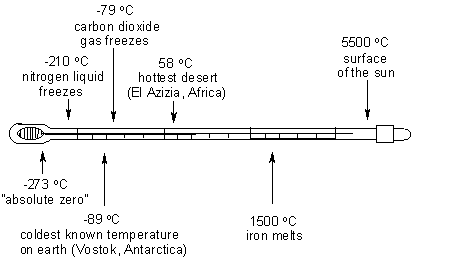
Temperature talk – how hot is what?
Have a look at our thermometer below. How much do you think the temperature varies where you live over a day, or between summer and winter, compared to these extremes?
 | 
