 Download this page as an activity sheet (pdf, 143KB). Download this page as an activity sheet (pdf, 143KB).
Go Green
In this activity, you can:
Please follow any safety instructions highlighted like this in red.
What is recycling?
"I've never understood why all those humans get rid of all the tastiest bits!"
Most people are familiar with recycling but often we forget it means more than just throwing the odd empty can of pop or glass bottle into a recycling bin. Recycling is a multi-stage process which involves many people and many different processes:
- People collect their recyclables and take them to collection points, or they sort their recyclables for a regular doorstep collection.
- Companies must sort and process the materials collected, clean them up and sell them to manufacturers for reuse to make new products.
- Finally, people must want to buy the new products made from recycled materials.
Recycling is a great thing to do, but it still uses energy and itself produces waste. We can all play our part in saving the environment by avoiding using excessive amounts of packaging and other disposable materials in the first place, then reusing and recycling the remainder where possible.
So, remember the 3 R's: Reduce, Reuse and Recycle.
What can you do to help?
You could try having a waste free lunch! Lunchtime can mean a lot of waste: plastic and paper bags, crisp packets, drinks cartons, yoghurt pots, napkins, etc.
Challenge your friends and teachers to have waste free lunches. If you work together you could reduce lunchtime waste to almost nothing. Set your class waste reduction targets and write a questionnaire to see how much disposable packaging and single serving products your friends, parents and teachers buy. If you use an electronic questionnaire, you could save even more paper!

Recycling plastics
Plastic is a man-made polymer, which is a really long molecule built from many small molecular units repeated and joined together.
It can take up to 700 years for plastic to decompose and disappear from our landfill sites or dumps. This makes the recycling of plastics particularly important. Six different types of plastic have been designed to be recyclable. If you live in an area where plastics are recycled, you'll need to be aware of the numbered plastic recycling logos. We illustrate these below, together with what the different plastics can be used to make:
 | Polyethylene terephthalate (PET) | for water and pop bottles |
 | High-density polyethylene (HDPE) | for milk bottles |
 | Polyvinyl chloride (PVC) | for medical tubes, blood bags |
 | Low-density polyethylene (LDPE) | for plastic bags |
 | Polypropylene (PP) | for ketchup bottles, yoghurt pots |
 | Polystyrene (PS) | for meat and fish trays |
How many examples of each type of plastic can you find? Write down the kinds of things made from each. Can you find any other plastic abbreviations?
Other types of waste – waste electronic equipment
Over 20 million computers became obsolete in 1998 and only 13% were recycled! Electronic circuit boards, batteries and the tube inside your TV set contain hazardous materials such as lead, mercury and chromium. If not disposed of properly, these toxins can leach out of landfill sites and be released into the environment. In just one year, over 50,000 tonnes of valuable materials were recovered from disposed electronics for recycling and reuse – including steel, glass, plastic and precious metals like gold.
So, next time you ask for a new computer, stereo or mobile phone, where will your old one go? Can it be recycled? Do you really need a new one?
Back to the top.
Digesting the facts about starch
In contrast to plastic, starch is a natural polymer increasingly used to make environmentally-friendly packaging materials. Starch consists of spiral chains of sugars and these chains can break down into small sugar molecules that dissolve in water. This natural breakdown, or biodegradation, is accelerated by substances known as enzymes (we say it is enzyme catalysed). The same digestion process begins as soon as we put starchy food into our mouths, as saliva contains just the right enzyme, amylase. You can find out more about enzymes here.
We can see this process in action using some iodine tincture. This is available from chemists' shops as an antiseptic for treating minor wounds. You only need a very small amount and it is cheap. Caution: the solution (in alcohol) is flammable. It will also stain skin and other surfaces and it will sting on cuts or broken skin. Use it carefully, with a dropper to add one drop at a time.
You can prepare a starch solution to experiment with by boiling some rice and retaining the water (please ensure you have adult supervision when handling boiling water). Allow it to cool and keep the top half of the liquid by pouring if off into a container. You can discard the lower portion, which contains the larger starch particles.
Place about 10-20 ml (1-2 dessertspoons) of your starch solution in a small glass and carefully add one drop of iodine tincture. What do you see? You should observe the instant formation of a deep blue colour with deep blue bits of starch floating around. This colour is caused by iodine
molecules trapped in the spiral amylose molecules of starch, like our diagram below:
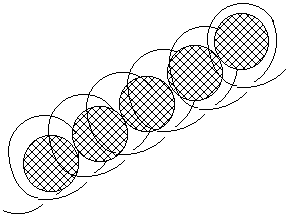
Now you can repeat the experiment, but this time add some saliva first by rolling a mouthful of water round your mouth for a while and adding this to your starch solution. Leave it
for a while before adding a drop of iodine solution. What do you see this time? You can
experiment by varying the amount of saliva used and the time elapsed before testing with iodine. The saliva contains the amylase enzyme – what is it doing to the starch?
Further investigations
You can test other foods (such as breads, rice grains and fruit) for starch by placing
them on a saucer and adding a drop of iodine solution. See if saliva reduces any blue/black staining.
Back to the top.
Twelve principles of green chemistry
Green chemistry is the pursuit of more environmentally friendly ways of making chemicals. Here are twelve principles which underlie green chemistry (don't worry, we don't expect you to understand all the jargon, they just illustrate what kind of issues green chemists have to think about!):
- Prevention
- It is better to prevent waste than to clean it up after it is formed.
- Atom economy
- Synthetic methods should be designed to maximise the incorporation of all materials used into the final product.
- Reducing hazards
- Wherever practicable, synthetic methods should use and generate substances of minimum toxicity to humans and environment.
- Safer chemicals
- Chemical products should be designed to preserve efficacy of function while reducing toxicity.
- Safer solvents/auxiliaries
- Use of auxiliary substances (solvents, separation agents, etc.) should be limited, and be innocuous when used.
- Energy efficiency
- Energy requirements should be minimised. Synthetic methods should use ambient pressure and temperatures.
- Renewable feedstocks
- A raw material or feedstock should be renewable rather than depleting, wherever technically and economically practical.
- Use fewer steps
- Unnecessary derivatisation (blocking group, protection/deprotection, temporary modification of physical/chemical processes) should be avoided wherever possible.
- Catalysis
- Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
- Pollution prevention
- Analytical methods are needed to allow for process monitoring and control prior to the formation of hazardous substances.
- Biodegradability
- Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment.
- Accident prevention
- Substances and the form of a substance used in the chemical process should be chosen so as to minimise the potential of chemical accidents, including releases, explosions and fires.
Adapted from Anastas, P. T.; Warner, J. C. 'Green Chemistry: Theory and Practice', Oxford University Press: New York, 1998.
Back to the top.
Recycling wordsearch
There are lots of kinds of solid wastes that can be recycled. Do you already recycle
some of the items on this list? Try to find them in the grid below (for a hint, roll your mouse over the wordsearch! – JavaScript required).
| Iron | Brass | Zinc | Lead | Office paper |
| Wood | Tin cans | Tyres | Cars | Aluminium can |
| Plastic | Concrete | Steel | Leaves | Motor oil |
| Copper | Gold | Metal | Rags | Newspaper |
| Paper | Jars | Glass |
| D | E | A | G | N | C | O | N | C | R | E | T | E | P | T |
| R | M | O | T | O | R | O | I | L | N | F | O | S | Y | C |
| U | W | F | Z | K | L | U | O | T | A | X | S | R | H | O |
| V | L | F | C | J | Z | D | G | W | C | I | E | T | D | R |
| R | T | I | N | C | A | N | S | L | M | S | I | A | A | R |
| E | S | C | O | P | P | E | R | N | U | M | B | C | E | U |
| P | H | E | G | X | O | Z | O | W | I | E | V | L | L | G |
| A | D | P | A | P | E | R | S | Q | N | T | A | T | L | A |
| P | L | A | S | T | I | C | S | W | I | A | J | L | Z | T |
| S | T | P | I | Z | U | H | T | O | M | L | M | A | W | E |
| W | B | E | C | U | B | D | E | O | U | S | I | H | R | D |
| E | G | R | A | G | S | T | E | D | L | N | D | P | Z | S |
| N | F | D | A | W | A | O | L | E | A | V | E | S | P | Q |
| C | E | O | X | S | U | H | A | J | C | H | C | A | R | S |
| E | C | T | A | S | S | A | L | G | F | T | Z | I | N | C | | 
