 Download this page as an activity sheet (pdf, 102KB). Download this page as an activity sheet (pdf, 102KB).
The Acid Test
In this activity, you can:
Please follow any safety instructions highlighted like this in red.
Acids and bases
What do lemons and vinegar have in common? They both taste sour on your tongue.
This is because they are acids and acids taste sour. Bases are the opposite of acids; they normally taste bitter and feel soapy. Lots of washing powders are bases ( WARNING: Do not taste these! ). Another word for a base is an alkali and we say that bases are alkaline compounds.
Some properties of acids and bases are:
- acids have a sour taste,
- acids are corrosive,
- acids lose their acidity when they are combined with bases and
- bases feel soapy.
Acids can sound really scary and some of them undoubtedly are, requiring very careful handling. As a precaution you should be wary of anything called an acid. However, there are a great many acids which are not only harmless to us but are vital to our survival.
 Did you know that Vitamin C is another name for a form of ascorbic acid? You'll find ascorbic acid together with another naturally occurring acid – citric acid – in orange and lemon juice. These are harmless partly because they are what's known as weak acids.
Acids react with bases and weak acids only really react with very strong bases. The problem with strong acids and strong bases is that they are so strong that they can always persuade even the weakest of bases or acids to react with them. There usually is something around to fill that role since all substances lie somewhere on a scale between being strong acids and being strong bases. Most natural substances lie in the middle zone where they're neither particularly acidic or basic.
Scientists use something called the pH scale to measure how acidic or basic something is. The pH scale measures acidity in the same way that a millimetre scale measures length, for example. A low pH indicates an acidic compound (an acid), whilst a high pH indicates a basic compound (a base). Here is an illustration of the pH scale from 0 (strongly acidic) to 14 (strongly alkaline) showing how acidic, or basic, some everyday things are:
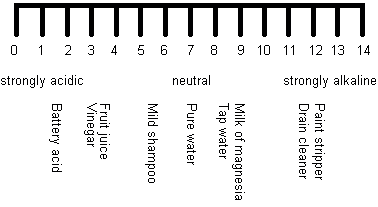
Things that are neither acidic nor basic are neutral and come in the middle of the pH scale – for example, pure water in the illustration above. Knowing whether something is acidic or basic can be very useful. For example, wasp stings are alkaline but bee stings are acidic. So if you get stung you need to put bicarbonate of soda on a bee sting and vinegar on a wasp sting. Hair conditioner is slightly alkaline as shampoo tends to be slightly acidic, so any traces of shampoo can be neutralised by the conditioner. What does this tell you about all-in-one shampoo/conditioners?
Back to the top.
How to make an indicator solution
"It's really weird, if you eat a lemon after a red cabbage, you completely change colour"
Special substances called indicators are used to determine whether things are acidic or basic by changing colour. You can make your own indicator from a red cabbage and find out which things in your kitchen are acidic and basic. To make your indicator solution, you need to:
- Cut the red cabbage up, put it into a saucepan and cover with boiling water. Please make sure you are supervised by an adult when handling boiling water.
- Stir it around and then leave it to soak for 15 minutes.
- Using a sieve, separate the liquid from the cabbage pieces.
- Keep the liquid in an old drinks bottle and label it clearly as INDICATOR.
To use your indicator solution to test things (the acid test!):
- Pour some of the indicator solution into several jars and label them.
- Write CONTROL on one of the jar labels. A control is a copy of an experiment in which you do nothing. It is used to show that results are due to the test and not to your equipment, allowing you to do a fair experiment. This jar will be your control, so you won't be adding anything to it – it's there so you can compare it to the others.
- To the other jars of indicator solution, add a few drops, or bits, of the things you want
to test. For example: lemon juice, cola, vinegar, a boiled sweet, washing up liquid, detergents,
bicarbonate of soda, shampoo and conditioner.
- Observe any colour changes in the jars, compared with the control. You can use this cabbage indicator pH scale to determine the acidity:
| pH | 2 | 4 | 6 | 8 | 10 | 12 |
| Colour | | | | | | |
| | red | purple | violet | blue | blue green | green |
Back to the top.
Invisible ink
You can even use the power of acid and base chemistry to write secret messages! You can write a message in invisible ink by doing this:
- Add 1 tablespoon of bicarbonate of soda (baking soda) to a mug of water.
- Dip a cotton-wool bud into the mixture and use it like a pen to write your
message onto a piece of paper. Handy hint: try not to put too much liquid onto the cotton bud!
- When the paper is completely dry, rub a red grape over the message. Handy hint: using a hair dryer speeds things up.
Can you work out what's going on? Of the bicarbonate of soda and the red grape, which do you think is acidic and which alkaline? |

