Biologically bound nickel in the plant matrix directs the selective hydrogenation transformation
Posted on 28 January 2022
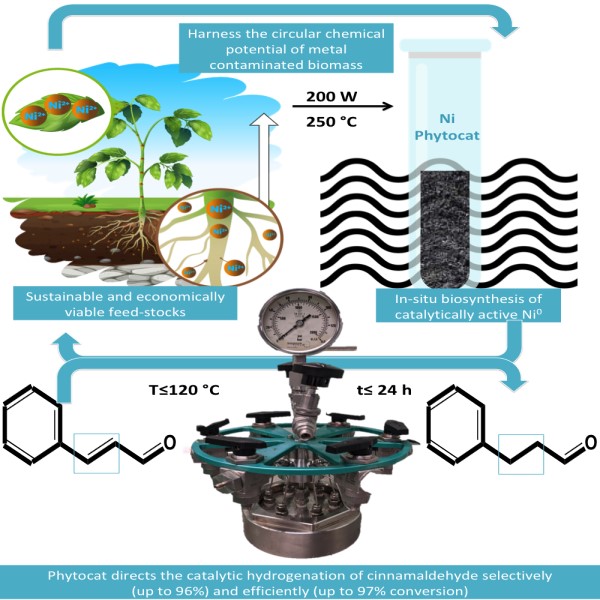
Nickel is a useful metal in areas such as chemical catalysis and batteries. However, it is also a problematic contaminant in many regions, including 5% of European agricultural land. Through a “double green” strategy, we have successfully used nickel captured by plants from contaminated soils as a catalyst for industrially important hydrogenation processes. Thus, toxic soils can be remediated, and at the same time, we can reduce the need for the wasteful and energy-demanding mining of virgin metal. We believe that by utilizing this green approach, we can develop more “phytocats” and involve different metals, especially where they can be used instead of scarce, difficult to refine and increasingly expensive noble metals.
There are several synthetic procedures for fabricating metal-based bio-catalysts. However, these protocols focus on the artificial incorporation of metallic species into bio-derived carbon materials. There are also several types of nickel catalysts that are active in reactions such as hydrogenations, but these present problems in addition to their need for virgin metals. Raney nickel, for example, is very active and inexpensive, but it is pyrophoric and difficult to handle. Conventional catalysts can also lack the desired selectivity in application. As an added bonus in the use of our phytocat in the hydrogenation of cinnamaldehyde, the natural encapsulation of nickel inhibits the undesired reduction of the carbonyl group.
Notes to editors:
This research paper is published in Applied Catalysis B: Environmental.
