Marine viruses inspire synthetic chemistry
Posted on 13 November 2019
Oceanic cyanobacteria are the most abundant oxygen-generating organisms on Planet Earth and are therefore extremely important to all life.
These organisms can be infected by viruses that play a part in a host of important biological processes, including photosynthesis.
In this new research, it has been found that enzymes isolated and repurposed from these viruses can also be used to add iodine atoms to of a diverse range of important organic molecules with remarkable selectivity.
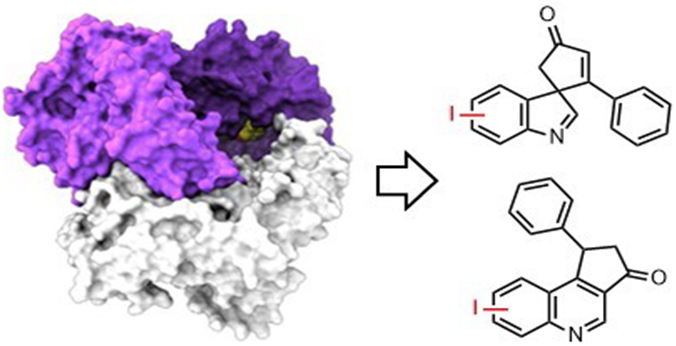
The discovery that important iodine-containing molecules can be made using these enzymes is expected to have numerous implications in the many fields that rely upon their synthesis. Halogenated compounds are widespread in the pharmaceutical and agrochemical industries. However, the reagents used to make them can produce hazardous waste and mixtures of compounds. Enzymes can provide a milder, more specific route to halogenated compounds, but so far the options for adding iodine have been very limited.
This ground-breaking research programme, largely funded by the ERC, Syngenta, and CRITICAT, has been led by Professor Rebecca Goss from the University of St Andrews, with Danai Gotski leading the bioinformatics and enzymology, Sunil Sharma driving forward the isolation and characterisation of the reactive products and Hannes Ludewig carrying out much of the structural Biology under the guidance of Jim Naismith.
Describing the discovery, Professor Goss said:
“We were absolutely blown away when we saw that far and away the preference was for iodination,” Goss says. “And it wasn’t iodinating just one or two substrates. It was iodinating a really broad sweep of structurally very diverse compounds, but in a highly selective manner.”
The York contribution to this project was to prepare some of the key organic molecules used to challenge the enzyme throughout this study. It was vital to test the enzyme against the widest possible range of diverse substrates, including our sterically demading spirocyclic compounds in order to demonstrate it could be an effective tool for late-stage diversification.
Dr Will Unsworth and Professor Richard Taylor said:
"We are delighted to have contributed to the success of this truly ground-breaking work in biocatalysis. It is wonderful to see how well the diverse molecules made here in York are tolerated by this remarkable enzyme."
This work has been published in Nature Chemistry.
