Dr Will Unsworth
Leverhulme Trust Early Career Research Fellow
Click for link to Group Webpages
Research
Novel methods to synthesise functionalised molecules: macrocycles, spirocycles, heterocycles and natural products
My group is interested in the development of new methods for the synthesis of functionalised, complex molecules with applications.
Current Research Interests: Macrocycle Synthesis, Direct Imine Acylation, spirocyclisation, C–H activation, alkyne activation, copper catalysis, rhodium catalysis, asymmetric synthesis, target synthesis. More details on current projects are included below:
Cascade Ring Expansion
End-to-end cyclisation reactions (1 to 2) to make medium-sized rings/macrocycles are typically inefficient. We believe that by designing reaction sequences such that overall end-to-end cyclisation is achieved via cyclisation/ring expansion cascades (3 to 4 to 5) a more kinetically favourable reaction course can be followed. This has been demostrated in our proof of concept study for the atroposelective synthesis of medium-sized rings 8. Expanding upon this idea will be a major research focus for the Unsworth group in the coming years.
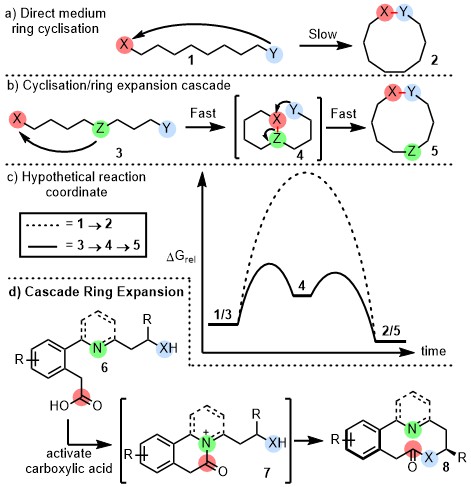
A. Lawer; J. A. Rossi-Ashton; T. C. Stephens; B. J. Challis; R. G. Epton; J. M Lynam; W. P. Unsworth, Angew. Chem., Int. Ed. 2019, 58, 13942–13947
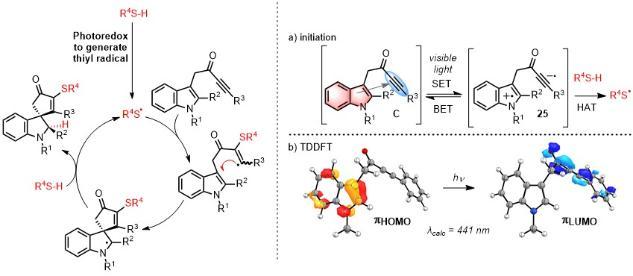
Photochemistry and radicals in synthesis
The Unsworth group has recently developed an interest in application of photochemistry and photoredox in synthesis in radical-based processes:
H. E. Ho, A Pagano, J. A. Rossi-Ashton, J. R. Donald, R. G. Epton, J. C. Churchill, M. J. James, P O'Brien, R. J. K. Taylor, W. P. Unsworth, Chem. Sci. 2020, 11, 1353-1360
A. K. Clarke, A. P. Parkin, R. J. K. Taylor, W. P. Unsworth, J. A. Rossi-Ashton, ACS Catal. 2020, 10, 5814-5820
Asymmetric Catalysis
The Unsworth group has a broad interest in catalysis, including asymmetric processes for the functionalisation of indoles:
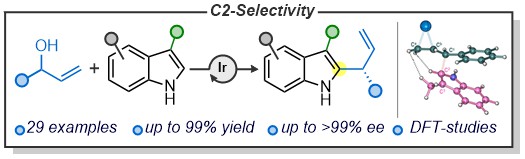
J. A. Rossi-Ashton, A.K. Clarke, J. R. Donald, C. Zheng, R. J. K. Taylor, W. P. Unsworth, S.-L. You, Angew. Chem., Int. Ed. 2020, 59, 7598–7604
Successive Ring Expansion (SuRE)
Important applications across the physical sciences rely on the synthesis of functionalised macrocycles. At present, macrocycles are typically made via the end-to-end cyclisation of a linear precursor, a notoriously difficult and unpredictable transformation; in particular, achieving macrocyclisation (1 → 2, Figure 1a) rather than dimerisation (1 → 3) is a major challenge. The most common strategy used to combat this is to perform the reactions at high-dilution but while successful in many cases, such protocols are generally highly substrate dependent and impractical for large scale synthesis.
Successive Ring Expansion (SuRE for short) is designed to improve the efficiency of macrocycle synthesis by completely avoiding end-to-end macrocyclisation. It is based on the sequential insertion of linear fragments into existing cyclic systems, by coupling a cyclic compound (4) to a linear fragment 5, which can thenrearrange (6), initiating ring expansion, forming a ring enlarged product 7. A key design feature is the replication of the functionality in the cyclic starter unit in the ring-expanded product (circled), as this means that the same coupling/ring expansion sequence can be repeated, allowing further iterations to be performed in the same way (7 → 9; 9 → 11). SuRE can incorporate a range of linear fragments and can theoretically be repeated indefinitely, meaning macrocycles of virtually any ring size and composition are potentially accessible.
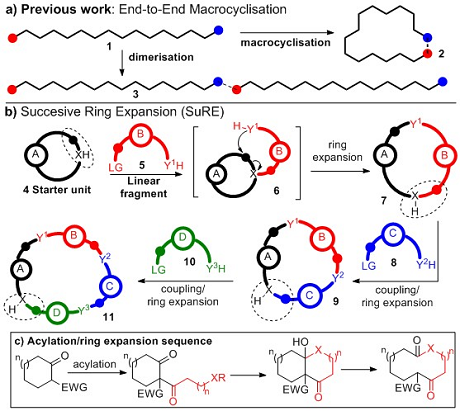
More deatils on this concept can be found in our first publication arising from this project, see: Angew. Chem. Int. Ed. 2015, 54, 15794 –15798. In this paper, the SuRE concept is validated using a telescoped two-step sequence, to generate macrocyclic lactams and lactones from cyclic β-keto esters (Figure 1c).
See also: Ring expansion approach to medium-sized lactams and analysis of their medicinal lead-like properties
L. G. Baud, M. A. Manning, H. L. Arkless, T. C. Stephens, W. P. Unsworth, Chem. Eur. J. 2017, 23, 2225.
Synthesis of Cyclic Peptide Mimetics by the Successive Ring Expansion of Lactams
T. C. Stephens, M. Lodi, A. Steer, Y. Lin, M. Gill, W. P. Unsworth, Chem. Eur. J. 2017 doi: 10.1002/chem.201703316
Catalyst Selective Synthesis
We are interested in the development of new catalytic processes in which multiple products can be generated from a common precursor through catalyst variation. This work has two major benefits: 1) by generating multiple products from a single starting material, the synthesis of potentially valuable compounds can be signifricantly streamlined; 2) we believe that challenging ourselves to uncover new catalyst selective synthetic methods is an effective way to inspire the discovery of new reactions and deliver new insight in catalytic processes.
The best illustration of this can be found in our 2016 publication entitled: Selective Synthesis of Six Products from a Single Indolyl α-Diazocarbonyl Precursor. The essence of work is in illustrated in the art work below; much as a single light source can be split into an array of colours with different visible properties, for example in a rainbow, we have been able to apply this concept chemically, to generate an array of 6 products with diverse chemical properties from a single precursor.
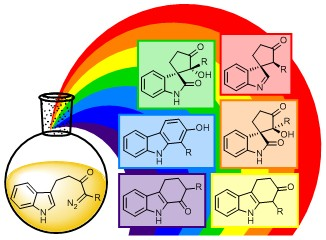
Dearomatising Spirocyclisation
We are interested in the development of new dearomatising spirocyclisation methods, especially those involving alkyne activation with π-acids and metal carbenoid intermedaites.
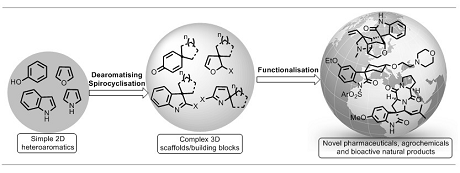
For selected publications, see:
-
J. T. R. Liddon, M. J. James, A. K. Clarke, P. O'Brien, R. J. K. Taylor, W. P. Unsworth, Chem. Eur. J. 2016, 22, 8777–8780.
-
M. J. James, R. E. Clubley, K. Y. Palate, T. J. Procter, A. C. Wyton, P. O’Brien, R. J. K. Taylor, W. P. Unsworth, Org. Lett. 2015, 17, 4372.
-
M. J. James, J. Cuthbertson, P. O’Brien, R. J. K. Taylor, W. P. Unsworth, Angew. Chem. Int. Ed. 2015, 54, 7640.
Publications
Selected recent publications (for a full list of 60, see the York Research database link in the right-hand column)
A. Lawer, R. G. Epton, T. C. Stephens, K. Y. Palate, M. Lodi, E. Marotte, K. J. Lamb, J. K. Sangha, J. Lynam, W. P. Unsworth, Chem. Eur. J. 2020, doi:10.1002/chem.202002164
A. K. Clarke, W. P. Unsworth, Chem. Sci., 2020, 11, 2876–2881
A. K. Clarke, A. P. Parkin, R. J. K. Taylor, W. P. Unsworth, J. A. Rossi-Ashton, ACS Catal. 2020, 10, 5814-5820
J. A. Rossi-Ashton, A.K. Clarke, J. R. Donald, C. Zheng, R. J. K. Taylor, W. P. Unsworth, S.-L. You, Angew. Chem., Int. Ed. 2020, 59, 7598–7604
J. A. Rossi-Ashton, A. K. Clarke, R. J. K. Taylor, W. P. Unsworth Org. Lett. 2020, 22, 1175–1181
H. E. Ho, A Pagano, J. A. Rossi-Ashton, J. R. Donald, R. G. Epton, J. C. Churchill, M. J. James, P O'Brien, R. J. K. Taylor, W. P. Unsworth, Chem. Sci. 2020, 11, 1353-1360
D. S. Gkotsi, H. Ludewig, S. V. Sharma, W. P. Unsworth, R. J. K. Taylor, M. M. W. McLachlan, S. Shanahan, J. H. Naismith, R. J. M. Goss, Nature Chem. 2019 doi: 10.1038/s41557-019-0349-z
A. Lawer; J. A. Rossi-Ashton; T. C. Stephens; B. J. Challis; R. G. Epton; J. M Lynam; W. P. Unsworth, Angew. Chem., Int. Ed. 2019, 58, 13942–13947
H. E. Ho, T. C. Stephens, T. J. Payne, P. O’Brien, R. J. K. Taylor, W. P. Unsworth, ACS Catal. 2019, 9, 504–510
A. K. Clarke, J. M. Lynam, R. J. K. Taylor, W. P. Unsworth, ACS Catal. 2018, 8, 6844
H. E. Ho, M. J. James, P. O’Brien, R. J. K. Taylor, W. P. Unsworth, Org. Lett. 2018, 20, 1439
- Dearomatizing Spiroannulation Reagents: Direct Access to Spirocycles from Indoles and Dihalides
J. T. R. Liddon, J. A. Rossi-Ashton, R. J. K. Taylor, W. P. Unsworth, Org. Lett. 2018, 20, 3349
T. C. Stephens, M. Lodi, A. Steer, Y. Lin, M. Gill, W. P. Unsworth, Chem. Eur. J. 2017, 23, 13314
W. P. Unsworth, J. R. Donald, Chem. Eur. J. 2017, 23, 8780
-
Ring expansion approach to medium-sized lactams and analysis of their medicinal lead-like properties
L. G. Baud, M. A. Manning, H. L. Arkless, T. C. Stephens, W. P. Unsworth, Chem. Eur. J. 2017, 23, 2225.
-
Selective Synthesis of Six Products from a Single Indolyl α-Diazocarbonyl Precursor
M. J. James, P. O’Brien, R. J. K. Taylor, W. P. Unsworth, Angew. Chem. Int. Ed. 2016,
-
J. T. R. Liddon, M. J. James, A. K. Clarke, P. O'Brien, R. J. K. Taylor, W. P. Unsworth, Chem. Eur. J. 2016, 22, 8777–8780.
-
The Synthesis of Structurally Diverse Macrocycles By Successive Ring Expansion
C. Kitsiou, J. J. Hindes, P. I’Anson, P. Jackson, T. C. Wilson, E. K. Daly, H. R. Felstead, P. Hearnshaw, W. P. Unsworth, Angew. Chem. Int. Ed. 2015, 54, 15794 –15798.
-
M. J. James, R. E. Clubley, K. Y. Palate, T. J. Procter, A. C. Wyton, P. O’Brien, R. J. K. Taylor, W. P. Unsworth, Org. Lett. 2015, 17, 4372.
-
M. J. James, J. Cuthbertson, P. O’Brien, R. J. K. Taylor, W. P. Unsworth, Angew. Chem. Int. Ed. 2015, 54, 7640.
Group

For up to date news, follow the Unsworth group on Twitter @UnsworthChem
Current Group Members
Dr Aimee Clarke (EPSRC funded PDRA)
James Rossi-Ashton (4th year PhD, co-supervised with Richard Taylor)
Wendy Robinson (3rd year PhD student, co-supervised with Gideon Grogan and Alison Parkin)
Kleopas Palate (3rd year PhD student, co-supervised with Peter O'Brien)
Ryan Epton (2nd year PhD student, co-supervised with Jason Lynam)
Rebecca Donovan (2nd year PhD student, co-supervised with Tom Farmer and Rob McElroy)
Balazs Pogranyi (2nd year PhD student, co-supervised with Gideon Grogan)
Zhongzhen Yang (1st year PhD student)
Nantachai Inprung (1st year PhD student, co-supervised with Richard Taylor and Michael James)
Verity Barber (1st year PhD student, co-supervised with Gideon Grogan)
Illya Zalessky (1st year PhD student)
Dominic Spurling (MRes)
Former Group Members
James Clegg (MChem 2019/20)
Ruben Godwin-Suttie (MChem 2019/20)
Youstena Hana (Erasmus)
Dr. Hon Eong Ho (EPSRC funded PDRA, co-supervised with Richard Taylor and Peter O'Brien)
Farhaan Dobah (visiting student from University of Cape Town)
Thomas Stephens (PhD student, co-supervised with Martin Fascione)
Chris Baldwin (MChem 2018/19)
Jess Hine (MChem 2018/19)
Angela Pagano (Erasmus+ student)
Dr Aggie Lawer (EPSRC funded PDRA, 2018)
Yun Lin (MRes)
Michelle Yeung (MRes)
Emilie Marotte (Erasmus student)
Emma Wright (MChem 2017/18)
Brad Challis (MChem 2017/18)
Dr. Mahendar Lodi (Indian-SERB funded PDRA 2016/17)
Jade Sangha (MChem 2016/17)
James Pitts (MChem 2016/17)
Andrew Steer (Research Associate, 2017, co-supervised with Alison Parkin and Gideon Grogan)
Leonard Himmel (Erasmus student 2017)
Laetitia Baud (Erasmus student 2016)
Morgan Manning (MChem 2015/16)
Helen Arkless (MChem 2015/16)
Christiana Kitsiou (PDRA, 2015)
Phil I'Anson (MChem 2014/15)
Jordan Hindes (MChem 2014/15)
Paula Jackson (MChem 2013/14)
Thomas Wilson (MChem 2013/14)
Visitors and placement students
Somchai Noppawan (2017/18)
Matthew Gill (2016)
Darius Stankevicius (2016)
Hannah Felstead (2015)
Eleanor Daly (2014)
Peter Hearnshaw (2013)
Awards and distinctions
Invited Talks and Seminars
2020
- LIMA (Laboratoire d'Innovation Moleculaire et Applications), ECPM (École européenne de chimie, polymères et matériaux), France, February 2020.
- ISIS (Institut de Science et d'Ingenierie Supramoleculaires), Universite de Strasbourg, France, February 2020
- ICCMO (Institut de Chimie Moléculaire et des Matériaux d'Orsay), Universite Paris-Sarclay, France, February 2020
- ICSN (Institut de Chimie des Substances Naturelles), Universite Paris-Sarclay, France, February 2020
2019
- NOST OCC-2019, Udaipur, India, December 2019
- Sheffield Hallam University, November 2019
- GSK, Emerging Academics Symposium, Stevenage, October 2019
- University of Manchester (Fellowship Advice Session for Postdocs in School of Chemistry), October 2019
- University of Manchester, October 2019
- 10th Eurasian Meeting on Heterocyclic Chemistry (EAMHC), Milano Marittima, Italy, September 2019
- University of Nottingham, September 2019
- Cardiff University, September 2019
- 26th International Symposium: Synthesis in Organic Chemistry, Cambridge, July 2019
- Young Chemists 2019 (YC19), Imperial College London, April 2019
- Northumbria University, April 2019
- University of Cape Town, South Africa, February 2019
- Stellenbosch University, South Africa, February 2019
- Syngenta, Jealott’s Hill, February 2019
- Lancaster University, February 2019
- University of Warwick, January 2019
- Evotec, January 2019
- Vertex, January 2019
- University of Bath, January 2019
2018
- Queen Mary, University of London, November 2018
- Heriot Watt University, October 2018
- RSC UK-India Symposium on Advances in Organic Chemistry, University of Oxford, September 2018
- Gregynog Synthesis Workshop, Sep 2018
- York 'Pint of Science', Walmgate Ale house, May 2018
- University of York, Athena SWAN 10 year Gold Celebration Event, May 2018
- University of Durham, 'Let's talk Fellowships' event, May 2018
- University of Leicester, April 2018
2017
- University of Strathclyde, October 2017
- Eli Lilly 50th anniversary Erl Wood celebration, October 2017
- Sanghai Institute of Organic Chemistry, China, September 2017 (x 2)
- Fudan University, China, September 2017
- University of Strathclyde, April 2017
- Institute of Organic and Macromolecular Chemistry of the University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Hans Knöll Institute, Jena, Germany, April 2017
- Durham University, February 2017
- Institute of Cancer Research, Chelsea. RSC heterocyclic 1 day meeting, January 2017
2016
- University of York, Biological Physics seminar series, November 2016
- Macrocycles Beyond-the-rule-of-5 medicinal chemistry conference, Fidelta, Zagreb, October 2016
- AstraZeneca, October 2016
- Gregynog Synthesis Meeting, September 2016
- Eli Lilly, August 2016
- University of Leeds, May 2016
2015
- Keele University, March 2015
- Bradford University, RSC NE regional meeting, April 2015
- Buzios, Brazil. BMOS meeting, November 2015.
- PUBlic seminar, York Brewery, York. (Public engagement talk)
2014
- May 2014, Zagreb, Macrocycles - synthesis, medicinal chemistry and biological activity.
- September 2014, Gregynog Synthesis Meeting
Awards
- Thieme Chemistry Journals Award 2020
- RSC Hickinbottom award, May 2018
- European Lead Factory Chemical Library Creativity Award, October 2017
- Dial-a-Moleucle/Directed Assembly AmigoChem Prize, September 2017.
- RSC/BMOS young investigator award, November 2015.
- Dave Kelly cup, Gregynog synthesis meeting, 2014
About Will
Biography

Will is originally from Coppull, near Chorley in Lancashire (UK). He studied chemistry at the University of Oxford, and remained there to complete his PhD studies in the group of Professor Jeremy Robertson. He completed his PhD in 2010 and then began work at the University of York, first as a postdoctoral research associate in the group of Professor Richard J K Taylor, before being appointed to a Research and Teaching Fellowship in 2013. In 2016 Will took up a Leverhulme Trust Early Career Fellowship to develop new procedures to synthesise functionalised macrocycles and is now the holder of the inaugrual Eleanor Dodson Fellowship at the University of York. His current research interests include ring expansion approaches for the synthesis of medium-sized rings and macrocycles, the construction of diverse spirocyclic scaffolds, cascade reactions and total synthesis. He now lives in York with his wife Hayley and two children Billy and Catherine.
Collaborators
External
- Prof Rebecca Goss (St Andrews)
- Prof Shuli You (Shanghai Institute of Organic Chemistry)
- Prof Chao Zheng (Shanghai Institute of Organic Chemistry)
- Dr Wade Petersen (University of Cape Town)
Internal (York) - Chemistry
Prof Richard Taylor, Prof Peter O'Brien, Prof Gideon Grogan, Prof Ian Fairlamb, Prof Jacqueline Hamilton, Dr Jason Lynam, Dr Alison Parkin, Dr Michael James, Dr Andrew Rickard, Dr Thomas Farmer, Dr C. Robert McElroy, Dr Charlotte Willans.
Internal (York) - other departments
Photos

Group photo 2017

Group Christmas meal 2016

Group Photo 2016 (Left to Right: Lin, Ton, James P, Leo, Will, Jade, James R, Michelle)

Gregynog Synthesis Meeting 2016

Will presenting his SuRE methodlogy at the BMOS meeting, Buzios, Rio de Janeiro, Brazil

Contact details
Useful links
