Professor Richard Taylor
Telephone: 01904 322543Email: richard.taylor@york.ac.uk
Taylor Group Website
The Total Synthesis of Compounds of Biological Importance:
The Design and Development of New Synthetic Methods
We are involved in two main areas of research which are often interlinked. The first is the synthesis of natural product and related compounds of biological interest. In order to carry out efficient target molecule synthesis, it is often necessary to develop new synthetic methodology. This leads on to the second main research area, the development of new synthetic transformations, particularly those using organometallic and organosulfur reagents, and new catalytic processes. We are also interested in the design of new telescoped and cascade organic transformations (including tandem oxidation processes, TOP) with environmental benefits.
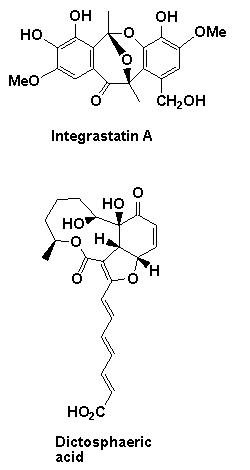
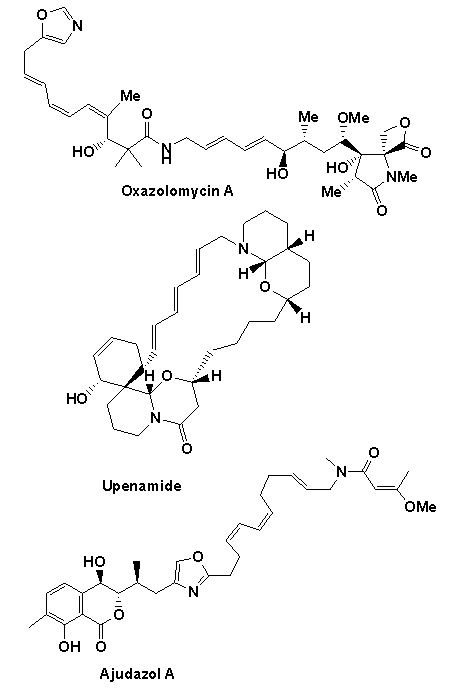
A number of natural product targets have been targeted using the above chemistry including the potent anticancer and antibiotic agents ajudazol A colletofragarone A, daphnezomine N, dictyosphaeric acid, grandisine B, integrastatin A, janoxepine, oxazolomycin A and 'upenamide (see below).
In terms of methodology the following areas are of current interest:
- The development of new Cu(II)-catalysed Ar-H activation routes to oxindoles and related heterocycles
- Convergent acyliminium methodology for diversity in heterocyclic scaffolds
- Dearomatising spirocyclisation procedures, especially those involving alkyne activation with π-acids
For a detailed description of the group’s activities please visit the Taylor Group web pages
3D view of (-)-grandisine B: A natural product synthesised in our group

