Haber, ethics and the Nobel Prize
Posted on 16 December 2016
Haber was awarded the Nobel Prize in 1918 for his work on ammonia synthesis. The process he developed – now known as the Haber process – fixes nitrogen from the air to make ammonia, which can be used to make synthetic fertilisers. However, some scientists have argued that Haber should never have been awarded the Nobel Prize.
The story of Fritz Haber and the use of synthetic fertilisers raise ethical questions about the impacts of scientists and their work on society and the environment.
The Haber process and synthetic fertilisers
The Haber process has been described as “the reaction that changed the world”, and today it is estimated that over a third of the world’s human population relies on food grown with synthetic fertilisers.
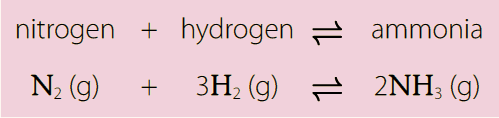
The Haber process requires a high energy input, which is primarily provided by burning fossil fuels, creating greenhouse gas emissions. The ammonia produced by the Haber process is used to make synthetic fertilisers. Nitrates from these synthetic fertilisers can pollute groundwater and drinking water, and they can bioaccumulate in food chains to toxic levels. They can also produce algal blooms in ponds and lakes, which damage aquatic ecosystems through eutrophication.
Fritz Haber, Clara Immerwahr and World War I
Scientists Fritz Haber and Clara Immerwahr were married from 1901 until her death in 1915.
(Images ©Emilio Segre Visual Archives/American Institute of Physics/Science Photo Library)
At the outbreak of World War I in 1914, Fritz Haber was put in charge of the development of raw materials for the German war effort. He was keen to do what he saw as his patriotic duty. As part of this work, he came up with the idea of releasing highly toxic chlorine gas into the enemy trenches – a kind of chemical weapon.
Haber was married to Clara Immerwahr, a German chemist. She was the first woman in Germany to be awarded a doctorate in chemistry, following her research into the solubility of metal salts. She went on to work as a laboratory assistant, which was the most senior position a woman was allowed to hold at that time. Immerwahr was disgusted by her husband’s work on chemical warfare. She apparently pleaded with him to stop but he would not – he regarded her protests as treasonous to her country. Immerwahr publically protested that the work of her husband was a “perversion of the ideals of science” and that it was “a sign of barbarity, corrupting the very discipline which ought to bring new insights into life”.
In April 1915 the first chlorine gas attack of the First World War was carried out in Flanders, Belgium. The attack was a direct result of Haber’s work and was supervised by him. Just 10 days later, Clara Immerwahr committed suicide.
Haber, ethics and the Nobel Prize
Fritz Haber was awarded the Nobel Prize for Chemistry in 1918, but this was controversial. The Nobel Prize committee recognised that although he had contributed to the creation of chemical weapons, his development of ammonia synthesis meant that he was a man who had given “the greatest benefit to mankind”. Not everyone agreed with the decision. It has been reported that the famous physicist Ernest Rutherford refused to shake Haber’s hand at the Nobel Prize award ceremony.
Science and technology are not separate from society. When deciding how scientific knowledge is used, we have to consider ethical questions related to social and environmental issues. Are all applications of science ethically acceptable? Ethical questions involve making decisions about what is right and wrong, and science alone cannot provide the answers.
To do
- Explain how the Haber process has made a significant positive difference to our lives.
- Describe unintended impacts of the Haber process on the environment.
- Summarise views for and against the award of the Nobel Prize to Fritz Haber.
- Discuss the following ethical questions:
- Do the benefits of synthetic fertilisers outweigh their damaging impact on the environment?
- Should scientists help to develop chemical weapons?
- Is a scientist responsible for all of the ways in which their work is used?
- Does Haber’s work on both ammonia synthesis and chemical warfare amount to "the greatest benefit to mankind”?
GCSE Twenty First Century Science specification links:
C6.4.1–3, C6.4.9, B3.4.1, B6.4.1, B6.4.3, IaS4.1–5
Blog post written by Alistair Moore from the Twenty First Century Science project at the University of York Science Education Group. Photograph of Nobel Prize medal taken by Alistair Moore at the Nobel Museum in Stockholm, Sweden.
This blog post was adapted from case study C7.3 “Are all applications of science ethically acceptable?” in the GCSE Chemistry student book for Twenty First Century Science (ISBN 978-0198359647), with kind permission of Oxford University Press. We are grateful to Helen Harden and Lynda Dunlop for their work on the original case study.
Copies of the student books and other Twenty First Century Science teaching and learning resources can be obtained from: https://global.oup.com/education/content/secondary/series/21st-century-science-3ed/?region=uk

