Professor Ian Fairlamb
01904 324091
Email: ian.fairlamb@york.ac.uk
See also Fairlamb Research Group website
Catalysis and Target-Oriented Synthetic Chemistry: Unlocking Reactivity and Selectivity Using Robotic Systems and Data Analysis
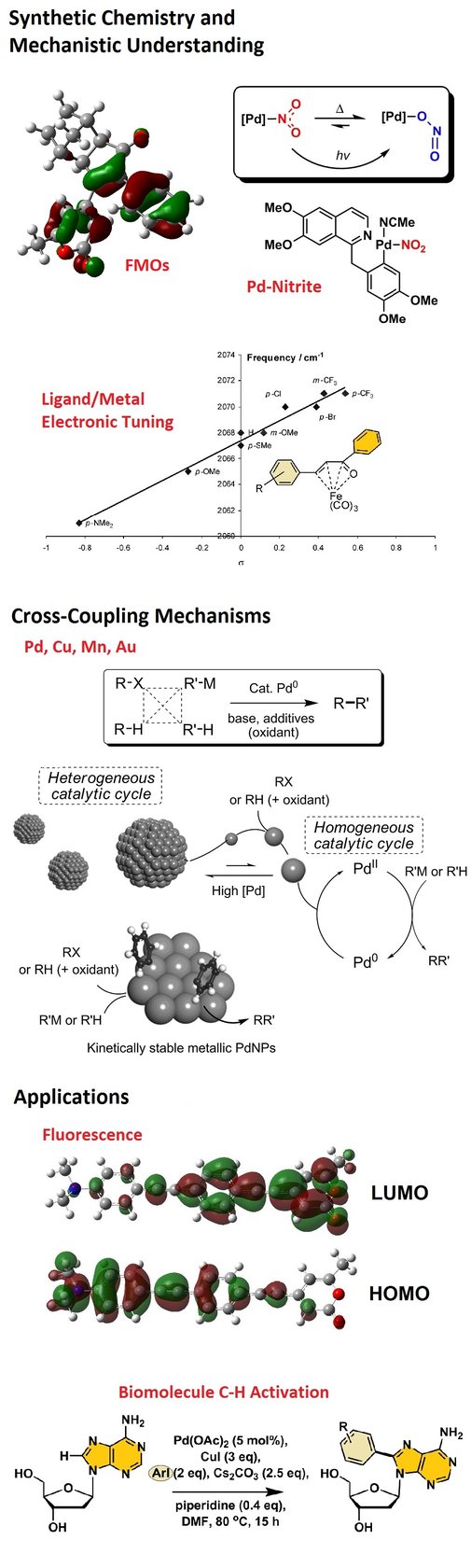
Our core interests are in the development and application of transition metal chemistry in mechanism-driven catalysis and synthetic chemistry. We are particularly interested in Pd, Fe and Mn catalyst design and mechanistic understanding, especially in C-H and C-X bond activation processes. Our work has strong links to the agrochemical, catalysis, life sciences and pharamaceutical sectors. Organometallic therapeutic carbon monoxide releasing molecules and fluorescent 'drug-like' heteroaromatic compounds are compound classes of interest. We use natural product frameworks as a sharpening tool for our new catalysts and catalytic processes. Quantitative mechanistic chemistry underpins both our fundamental and applied research. We utilise automated robotic systems to aid with reaction screening and understanding catalyst activation and deactivation pathways, which is important to applied synthetic chemistry. The work involves principal component analysis and decision tree learning, and holistic understanding of reaction outcomes.
Selected Publications
- Mn(I) carbonyl catalysis and C-H bond activation
J. Am. Chem. Soc. 2021, 143, 1356; Chem. Eur. J. 2021, 27, 3979; J. Am. Chem. Soc. 2019, 141, 2316, Chem. Commun. 2019, 55, 3211; Nature Catal. 2018, 1, 830; Angew. Chem. Int. Ed. 2016, 55, 12455. - Cross-coupling, C-H activation: Involvement of Pd3 clusters and Pd nanoparticles
Green Chem. 2021, 23, 920; Chemical Science 2019, 10, 7898; Chem. Commun. 2015, 51, 16289; Angew. Chem. Int. Ed. 2010, 49, 1820.
- On impurities in Pd(OAc)2/nitrite linkage isomerisation at Pd and Pd-NOx redox activity in C-H bond activation
J. Am. Chem. Soc. 2017, 139, 1177; Angew. Chem. Int. Ed. 2015, 54, 10415; Chemical Science 2012, 3, 1656.
- Catalysis with Au-NHC, Au-NHC nanoparticles; Cu-NHC complexes and C-H bond functionalization
Chem. Commun. 2016, 52, 5057; New. J. Chem. 2009, 33, 1837; J. Org. Chem. 2009, 74, 5810.
- Total synthesis of phacelocarpus 2-pyrone A - application of AsCat, imidate ligand effects
Chem. Eur. J. 2015, 21, 18905; Chem. Commun. 2015, 51, 3466.
- Biocatalysts for the synthesis of pharamaceutically-relevant amides
Angew Chem. Int. Ed. 2018, 57, 11584; ACS Catal. 2020, 10, 4659.
- π-Acidic alkene ligand effects at Pd, including dibenzylidene acetone (dba) ligands
J. Am. Chem. Soc. 2013, 135, 8388.
- C-H bond activation in tryptophan containing compounds and aryl/heteroaryl diazonium salts
Org. Lett. 2020, 22, 7257; ACS Catalysis 2017, 7, 5174; Chem. Commun. 2014, 50, 3052.
- Pericyclic processes, 6π-electrocylization and Pauson-Khand chemistry
CrystEngComm 2012, 14, 5564; J. Org. Chem. 2011, 76, 5320 and Org. Biomol. Chem. 2008, 6, 4523.

Research
Overview
We are an Organic Chemistry research group with multi-disciplinary links to Computational Studies (DFT), Rich Data Analysis, Inorganic Chemistry and Nanoscience.
Projects
Industrially-relevant academic research programmes.
Use of automated chemical synthesis (robotics) and rich data analysis of reaction outcomes.
Mn-Catalysed C-H activation in organic chemistry.
Study of Pd clusters and nanoparticles in C-H activation chemistry.
Understanding mechanisms in Pd catalysis: towards sub 10ppm cross-coupling catalysis.
Catalytic C-H activation in biomolecules.
Research group(s)
The research group is made up of PhD students, post-doctoral scientists, M.Chem. students and research visitors. The group size fluctuates from year to year, typically having between 10 and 16 members.
Collaborators
Current York collaborators:
- Jason Lynam
- Julie Wilson
- Simon Duckett
- Peter O'Brien
- Robin Perutz
- John Slattery
- Gideon Grogan,
- Jane Thomas-Oates and
- Ed Bergstrom.
Current External collaborators:
Available PhD research projects
Please contact Professor Fairlamb about new research projects available within the group.