Professor Peter O'Brien
01904 322535
Email: peter.obrien@york.ac.uk
Development of New Methods in Synthesis and Medicinal Chemistry
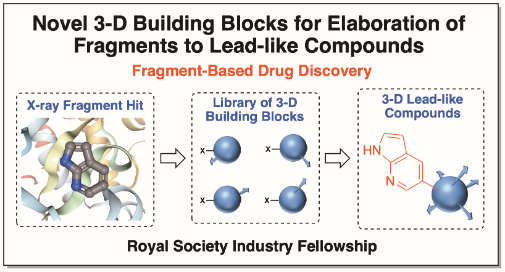
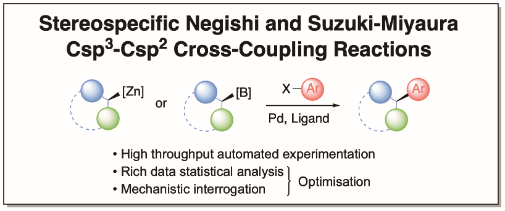
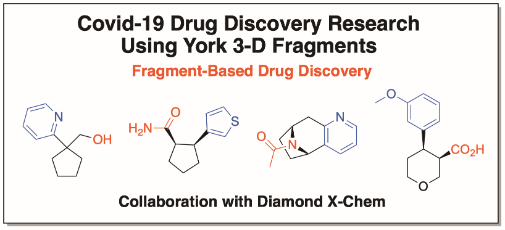
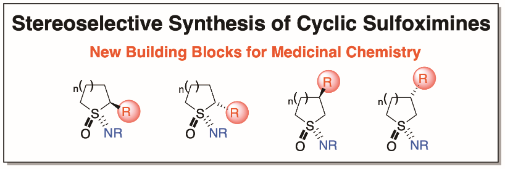
The O’Brien group’s research focuses on the development of new methods for organic synthesis. Projects in the group aim to develop novel, selective and synthetically useful methods that are technically simple, high yielding and robust. Historically, the group has studied asymmetric and mechanistic organolithium chemistry in detail. More recently, efforts have included extension to stereospecific Negishi and Suzuki-Miyaura sp3-sp2 cross-coupling reactions. In all projects, we apply the newly developed methodology to the synthesis of common motifs in blockbuster pharmaceuticals. We have ongoing interests in the design and synthesis of novel 3-D fragments for use in fragment-based drug chemistry, as well as developing new synthetic approaches for fragment elaboration. In collaboration with the Diamond X-Chem facility in Oxford, York 3-D fragments have been identified as useful starting points for drug discovery against two Covid-19 proteins. Another area of interest for the group is functionalised cyclic sulfoximines for use as medicinal chemistry building blocks.
Selected recent publications:
-
Fragment binding to the Nsp3 macrodomain of SARS-CoV-2 identified through crystallographic screening and computational docking
Sci. Adv. 2021, 7, eabf8711
-
Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease
Nat. Commun. 2020, 11, 5047
-
Design and Synthesis of 56 Shape Diverse 3-D Fragments
Chem. Eur. J., 2020, 26, 8969
-
Visible-light-induced intramolecular charge transfer in the radical spirocyclisation of indole-tethered ynones
Chem. Sci. 2020, 11, 1353
-
Enantioselective Lithiation–substitution of nitrogen-containing heterocycles
Org. React. 2019, 100, 255
-
Merging π-Acid and Pd Catalysis: Dearomatizing Spirocyclization/Cross-Coupling Cascade Reactions of Alkyne-Tethered Aromatics
ACS Catal., 2019, 9, 50
- Lead- and Fragment-oriented Synthesis, in Chemical and Biological Synthesis: Enabling Approaches for Understanding Biology, 2018, pp. 74-113
-
Gram-Scale Synthesis of the (−)-Sparteine Surrogate and (−)-Sparteine
Angew. Chem. Int. Ed. 2018, 57, 223
-
Increase of enzyme activity through specific covalent modification with fragments
Chem. Sci., 2017, 8, 7772
-
Silica-Supported Silver Nitrate as a Highly Activating Dearomatising Spirocyclisation Catalyst: Synergistic Alkyne Activation by Silver Nanoparticles and Silica
Angew. Chem. Int. Ed. 2016, 55, 13798
-
Selective synthesis of six products from single indolyl α-diazocarbonyl precursors via catalyst variation
Angew. Chem. Int. Ed. 2016, 55, 9671
-
Synthesis of Enantiopure Piperazines via Asymmetric Lithiation-Trapping of N-Boc Piperazines: Unexpected Role of the Electrophile and Distal N-Substituent
J. Am. Chem. Soc., 2016, 138, 651
-
Silver(I) or copper(II)-mediated dearomatisation of aromatic ynones: direct access to spirocyclic scaffolds
Angew. Chem. Int. Ed., 2015, 54, 7640

