Professor Paul Walton
Email: paul.walton@york.ac.uk
Research: Bioinorganic chemistry
Lytic Polysaccharide Monooxygenases (LPMOs), the histidine brace, signal-strapping and anglerases
LPMOs, along with other enzymes, have a copper-containing active site in an N-terminal histidine (Proc. Nat. Acad. Sci., 2011) coordinates to the copper. The active site was discovered in 2011 and is known as the histidine brace. LPMOs catalyse the oxidation of recalcitrant polysaccharides such as cellulose. Our current research interests involve understanding the catalytic mechanisms of these enzymes using a combination of structure, spectroscopy and theory, J. Am. Chem. Soc. 2023, and also revealing the wider role of LPMOs in biology, Science 2021, 774-779.
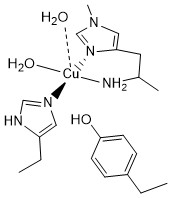
Signal-strapping and anglerases
In our recent study published in Nature Communications 2025, we introduce a novel sequence‐search strategy termed “signal-strapping”, which systematically targets secreted proteins bearing an N-terminal histidine (or other metal-chelating residue) to uncover previously unrecognized metalloproteins. Starting with known signal peptides, we appended a histidine and used this motif to search proteome databases, then validated several newly discovered protein families biochemically and structurally. Notably, we characterised four classes of bacterial metalloproteins—two of which we designate “anglerases,” embedded in metal‐uptake systems and likely functioning as metal-ion scavengers. Our findings reveal a hitherto under-appreciated mechanism by which bacteria may capture transition metals, and demonstrate the power of sequence-based heuristics to accelerate metalloprotein discovery.
LPMOs shown to have protective charge transfer pathway
Working with colleagues at the University of Manchester, we were able to determine the role of the active site tyrosine found in many LPMOs, J. Am. Chem. Soc. 2023. Using a combination of stopped flow spectroscopy, freeze-quench, EPR spectroscopy and HERF-D X-ray absorption spectroscopy, we demonstrated that the tryosine is oxidised during hydrogen-atom transfer to the histidine brace active site, which itself had become damaged during uncoupled turnover of the enzyme. As such, the tyrosine provides a means of protecting the active site of LPMOs from inadvertent oxidation. This work is related to earlier work we did on the formation of tyrosine radicals in LPMOs (J. Am. Chem. Soc. 2019).
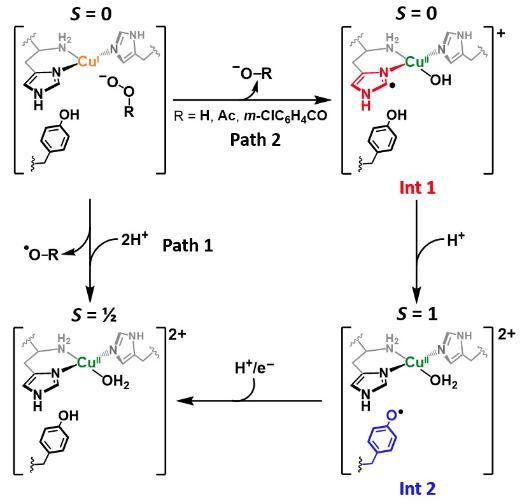
Figure. Reaction pathway for formation of protective tyrosyl radical in LPMOs
Recent publications
- Signal-strapping as a protein-sequence search method for the discovery of metalloproteins, J P L Franco Cairo, T L R Correa, W A Often, A K Nairn, J Walton, S T Sweeney, G J Davies, P H Walton, Nature Commun., 2025, 16, 9244. DOI: https://doi.org/10.1038/s41467-025-64309-x
- Secreted pectin monooxygenases drive plant infection by pathogenic oomycetes, F Sabbadin, S Urresti, B Henrissat, A O Avrova, L R J Welsh, P J Lindley, M Csukai, J N Squires, P H Walton, G J Davies, N C Bruce, S C Whisson, S J McQueen-Mason, Science, 2021, 373(6556), 774-779. DOI: 10.1126/science.abj1342
- Mapping the Initial Stages of a Protective Pathway that Enhances Catalytic Turnover by a Lytic Polysaccharide Monooxygenase, , J. Am. Chem. Soc. 2023, 145(37), 20672-20682 (open access article). DOI: https://doi.org/10.1021/jacs.3c06607
Biography
Paul Walton obtained his PhD in 1990, followed by two years as a NATO/SERC postdoctoral fellow at the University of California, Berkeley, USA. He joined the Department of Chemistry at York as a faculty member in 1993. Between 2004 and 2010 he was chair of department. His main research area is bioinorganic chemistry, in which he has made contributions to the understanding of copper oxidases, including the discovery of the histidine brace, the bioinformatic method of signal-strapping and anglerase metalloproteins. Paul is an internationally-known advocate of equality in sciences and lectures widely on the subject.
He is the recipient of multiple national and international awards, including:
Elected Foreign Member of the Royal Swedish Academy of Sciences, 2026.
Research: Gertrude Cropper Award, RSC's Joseph Chatt Award, IChemE's Global Energy Award, RSC's Rita and John Cornforth Award, University of Chalmers Jubilee professor 2020.
Teaching: RSC's Higher Education Teaching Award, Vice-Chancellor's Teaching Award.
Equality: RSC Inclusion and Diversity Prize, 2025, Royal Society's inaugural Athena Prize (runner-up). WISE man of the year shortlist.
He has also been Editor of Dalton Transactions (2004-2008), chair of Heads of Chemistry UK, chair of the Royal Society of Chemistry's Diversity Committee, was named as a 'Person of Influence' by the University of Toronto's Women in Chemistry Group and is one of the RSC's 175 Faces of Chemistry.

