Professor Andrew Parsons
+44 (0)1904 322608
Email: andy.parsons@york.ac.uk
Andy has a background in synthetic organic chemistry, with a particular focus on the synthesis of organic compounds using new radical reactions.
He has a wide-ranging interest in radical reactions, so, apart from their use in the synthesis of small molecules, he is also interested in polymerisation processes and natural oxidation reactions.
In 2005, Andy was awarded the Royal Society of Chemistry Higher Education Teaching Award. The same year he was awarded a Department of Chemistry Teaching Prize (2005), in 2006 a Vice Chancellor’s Teaching Award, in 2008 a University ‘Making the Difference‘ award, and in 2011, as Head of our Admissions Team, a VC Silver Award for Outstanding Achievement in the Excellence Category.
In 2011 the team was also shortlisted for the Outstanding Student Admissions Team at the Times Higher Education (THE) Leadership and Management Awards. In 2016, in the (former) YUSU (University of York Student Union) Awards, he was awarded Highly Commended in the ‘Most Enthusiastic’ category.
More recently, in 2018, Andy was awarded a prestigious National Teaching Fellowship by the Higher Education Academy in reconition of his inspirational and innovative approaches to teaching.
As Deputy Head of Department (2008-16), he had wide-ranging responsibilities including overseeing a £29 million investment in new Chemistry buildings, including contributing to the design of our extensive undergraduate teaching labs, aimed at setting the UK standard for practical chemistry.
![]()
He is the author of three undergraduate textbooks including Keynotes in Organic Chemistry (Wiley) and Chemistry3 (OUP), which is the leading undergraduate Chemistry textbook for first year students.
He is a Fellow of the Royal Society of Chemistry, a former Head of Vanbrugh College (2016 to 2018) and, in 2017, achieved the status of Senior Fellow of The Higher Education Academy.

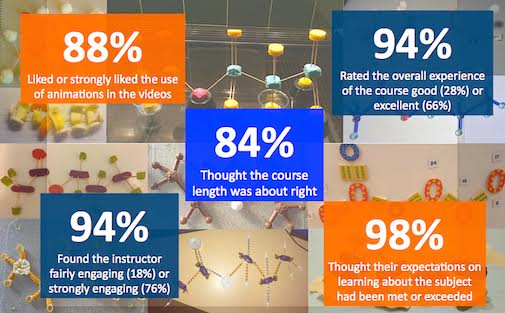
In January 2017 he delivered a first ever University of York MOOC, called Exploring Everyday Chemistry, comprising four weeks of learning with over 85 individual learning steps, including more than 30 videos and screencasts. Following re-runs, the course has attracted over 23,000 learners from around 150 countries.
In November 2019 Andy delivered a TEDx talk explaining how chemistry can help us orchestrate a brighter future.
Recent publications
Retrosynthesis analysis in action: The effectiveness of filling in the gapsA F Parsons, J. Chem. Educ., 2021, 98(10), 3390Organic Fanatic: A quiz-based mobile application game to support learning the structure and reactivity of organic compoundsJ Shoesmith, J D Hook, A F Parsons and G A Hurst, J. Chem. Educ., 2020, 97(9), 2314Exploring Everyday Chemistry: The effectiveness of an organic chemistry massive open online course as an education and outreach tool
A F Parsons, J. Chem. Educ., 2020, 97(5), 1266Going green in process chemistry: Optimizing an asymmetric oxidation reaction to synthesize the anti-ulcer drug esomeprazoleG D McAllister and A F Parsons, J. Chem. Educ., 2019, 96(11), 2617Flipping introductory retrosynthetic analysis: An exemplar course to get the ball rollingA F Parsons, J. Chem. Educ., 2019, 96(4), 819MOOCs as ‘chemical attractants’A Parsons and I Barr, Waikato Journal of Education, 2018, 23(2), 5Students don’t appreciate what chemistry can doA F Parsons, Educ. Chem., 2018https://eic.rsc.org/opinion/students-dont-appreciate-what-chemistry-can-do/3009186.articleBreathe of lifeA F Parsons, Chemistry Review, 2018, 28, 2Wanted: Chemistry’s next superstarA F Parsons, Chemistry World, 2018https://www.chemistryworld.com/opinion/wanted-chemistrys-next-superstar/3009391.articleMassive open online courses (MOOCs)A F Parsons, Chemistry Review, 2017, 27, 14An app for applicantsG A Hurst, A F Parsons, K Sayer, J D Hook and C Fulford. FORUM Learning and Teaching Magazine, 2017A Mooc with no gobbledegookA F Parsons, Educ. Chem., 2017https://eic.rsc.org/opinion/a-mooc-with-no-gobbledegook/3007729.articleAn interdisciplinary summer for interdisciplinary studentsG A Hurst, B Garrett, J Webb and A F Parsons. FORUM Learning and Teaching Magazine, 2016https://www.scribd.com/document/330391856/Student-Work-UoY-Forum-41-Autumn-2016