Food security, traceability and authentication
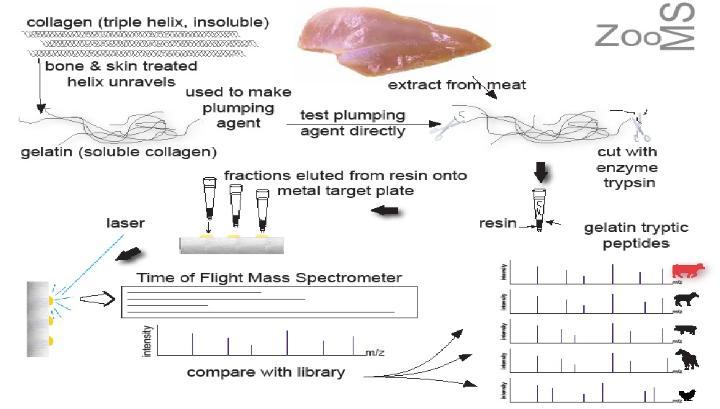
The protein collagen is abundant in bone and has extraordinary stability due to its three-dimensional structure. Jane Thomas-Oates (Chemistry) and Matthew Collins (Archaeology) have demonstrated that, contrary to received wisdom, collagen includes variations in its amino-acid sequence that are species specific.
In this research, collagen was extracted from very small samples of bone and analysed by mass spectrometry, allowing identification of the animal species from which the bone came. The same approach may be used for the detection and analysis of gelatin, which is produced from the collagen in bone on boiling. York’s analytical methods have been applied in food authentication, traceability and safety and have been shown to be effective even when the DNA has been destroyed. These techniques also identify collagen fragments present in gelatin-based plumping agents that retain water in meats for human consumption. Dr Julie Wilson (Chemistry and Mathematics) joined the team, applying data analysis and pattern recognition methods, to provide a rapid means for identification of the species of origin from the spectra. York’s authentication of food revealed contamination of chicken with pork-derived plumping agents, an issue particularly affecting communities with Halal and Kosher diets (about five million people in the UK choose to avoid pig and cow products).
These results have been disseminated by high-profile media reporting, including a one-hour BBC special, and the press. The Food and Environment Research Agency (FERA, an agency of the Department of the Environment, Food and Rural Affairs) has validated the York analytical method and applied it to processed food and pharmaceutical products. The results were highlighted in the press in 2009 and the debate over food authentication exploded in 2013 highlighting the economic effects of mislabeling. This research therefore has impact on public and commercial services as well as public debate. Gelatin (PDF ![]() , 644kb)
, 644kb)