![]()
![]()
![]()
Photocatalysis
Photocatalysts are a class of materials that mediate chemical reactions using photons as a source of energy. There are many potential uses including the degradation of chemicals detrimental to the environment and the conversion of solar into chemical energy. We are interested in the later application, specifically the production of dihydrogen and dioxygen from water that provides the basis for a clean fuel source with zero carbon emissions. Simplistically the concept is shown in figure 1. A photon is absorbed by a semiconductor causing electron excitation from the valence to conduction band. The excited electron can be used for reduction chemistry and the hole for oxidation chemistry. Many materials exhibit exciton (an electron-hole pair) formation that can be used for useful chemical reactions. However, significant challenges remain dependent on the application, including efficiency, photocatalyst stability and the use of visible (low energy) photons present in the solar spectrum. It is therefore necessary to identify and study new photocatalytic materials.
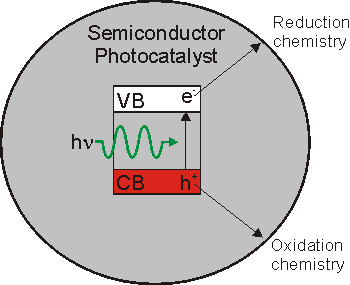
Fig. 1
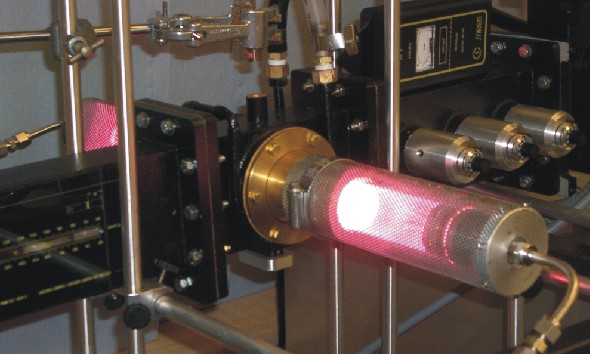
Microwave heating is a well established technique for solution based synthetic chemistry and most modern molecular laboratories now contain a microwave reactor. Commonly, rapid reaction rates can be achieved because of superheating that is a result of coupling between the solvent (or reactants) and microwave radiation. Similarly in the solid state one of the components of a reaction must couple with the microwaves, generating heat via dielectric or conduction losses to drive a reaction. Solids can heat at enormous rates (100 K/s), however many of interest do not couple with microwaves at room temperature limiting the application of this technique to preparative materials chemistry.
We have designed and constructed a reactor capable of initiating and sustaining microwave-induced plasmas of various gases including Ar, N2, H2/N2, NH3 and O2. The plasma can be used as a source of heat to drive bulk solid state reactions and also as a source of reactive species for gas-solid reactions for surface or bulk modification.

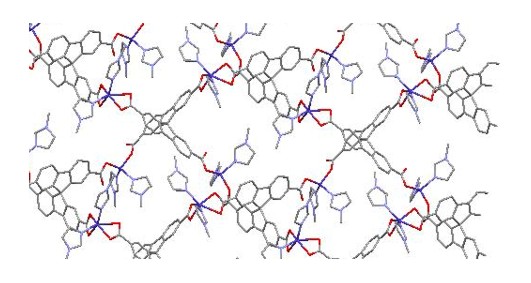
Molecular Chemistry
N-Heterocyclic Carbenes