Professor Kirsty Penkman
01904 322574
Email: kirsty.penkman@york.ac.uk
Analytical and Environmental Geochemistry, Biomolecular Archaeology
Member of BioArCh group
My research interest is in developing and applying analytical chemistry to archaeological and geological questions. Working collaboratively with a a wide range of disciplines, my focus is on the analysis of biomineral organics: their pathways of degradation, their methods of preservation, of detection, and how these molecules can inform us of an organism’s life and death history. I run the NERC-recognised amino acid dating facility, NEaar.


Dating the last 2.5 million years
One key problem is dating in the Quaternary (the last 2.5 million years), a crucial period for geological understanding with impacts on both climate change and human evolution. A well-constrained chronology is essential in almost all aspects of Quaternary research, but beyond the limit of radiocarbon dating (~50,000 years) there is no technique that can be applied ubiquitously in the terrestrial environment.
In recent years advances have been made in amino acid geochronology (Penkman et al., 2008 Quat. Geo; 2011, Nature), combining the isolation of an 'intra-crystalline' fraction of amino acids by exhaustive bleach treatment of ground shell carbonate (Sykes et al., 1995) with a new reverse-phase high pressure liquid chromatography (RP-HPLC) method for chiral amino acid analysis (Kaufman & Manley, 1998). This combination of techniques results in the analysis of D/L values of multiple amino acids from the chemically-protected protein within the biomineral, enabling both decreased sample sizes and increased reliability. The intra-crystalline protein occurs within a 'closed system' during the burial history of the shell, vital for the application of this technique for dating. Amino acid data obtained from the intra-crystalline fraction of calcitic biominerals (Bithynia opercula) indicate this to be a particularly robust repository for the original protein (Penkman et al. 2011, Nature), confirming the antiquity of the early human occupation of northern Europe (Parfitt et al., 2005, Nature).
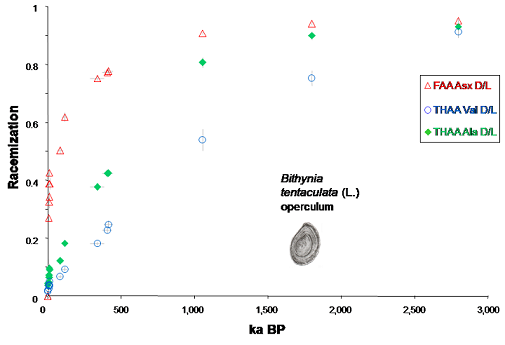
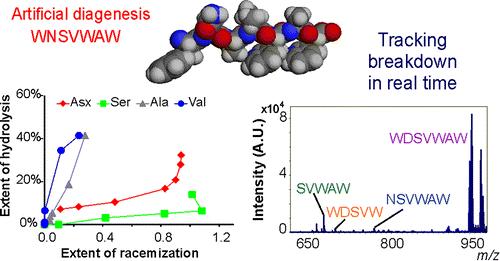
This intra-crystalline protein decomposition (lcPD) in closed system biominerals not only improves the precision and accuracy of amino acid geochronology, but also allows us to understand and test protein decomposition kinetics and mechanisms (Demarchi et al., 2013, An. Chem). Extension of the dating method from calcium carbonate materials such as corals (Hendy et al., 2012, GCA) and eggshell (Dermarchi et al., 2016, eLife) to calcium phosphate (tooth enamel; Dickenson et al., 2019, Quat. Geo.) has been an exciting breakthrough. We are now developing chronologies using these methods on a range of different fossils across the globe; please see my highlights page for links to our projects.
Understanding environmental change?
The study of fossil protein also has potential for use as an environmental proxy: our research includes analysing the proteins from speleothems and Greenland ice cores, assessing the microbial contribution to aquatic systems and isolating the stable isotopes of the ‘closed-system’ protein for palaeoclimate studies. We investigate the preservation and degradation of archaeological materials in situ (High et al., 2016, PNAS) and work closely with stakeholders such as Historic England (High & Penkman, 2020). We are also trying to understand the intimate relationship between the organics and inorganics in biomineral formation (Kim et al., 2016, Nature Materials; Kellock et al., 2020, Scientific Reports), including investigations into how corals respond to increasing CO2 concentrations.

Fossil molecules
A continuous focus of our research has been in ensuring the highest quality data from fossil samples, whose low concentrations of original organics are easily subject to modern contamination. The analytical difficulties of recovering the very low concentrations of useful biomolecules from fossil material require development and refinement, from intensive studies into the best methods of preparation, to the optimization of analytical methods by RP-HPLC, GC, soft ionisation protein mass spectrometry, FTIR and electron microscopy. This combination of complementary approaches has resulted in key evolutionary insights (Presslee et al., 2019, Nat Eco & Evo; Cappellini et al., 2019, Nature; Welker et al., 2020, Nature).