Professor Paul McGonigal
Email: paul.mcgonigal@york.ac.uk
Website: mcgonigalgroup.com
ORCiD: 0000-0002-8538-7579
Paul McGonigal is a Professor in Functional Organic Materials and EPSRC Fellow. He investigates dynamic process in organic functional materials with emphasis on exploiting stabilised, highly substituted aromatic carbocations, fluxional carbon cages, and supramolecular chemistry. Students in the group are exposed to a wide range of techniques in organic synthesis, advanced spectroscopy, organometallics, electrochemistry, and DFT modelling.
Please see our group web page for regular updates and news about the McGonigal Group's research, publications and group members.
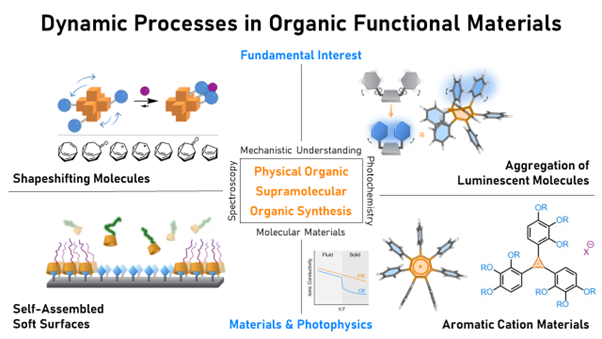
A graphical overview of the research areas investigated by the McGonigal Group
Shapeshifting Molecules
Different sequences of atoms give molecules with distinct shapes. This shape is key to a molecule’s properties, eg, its biological effect when binding proteins in our bodies. Conventionally, the atomic sequence of a molecule is fixed. We research fluxional carbon cages that have dynamic C–C bonding, giving them ‘shapeshifting’ properties. One advance in this area has been to develop rigid cage molecules that flip rapidly between mirror image forms (R and S stereochemistry). We are investigating how this dynamic stereochemistry interfaces with catalytically active metal as part of organometallic complexes.
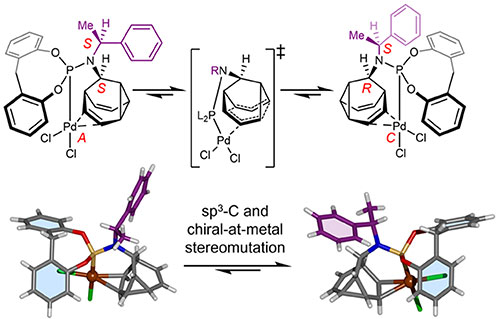
A fluxional barbaralane ligand passes its dynamic stereochemistry onto to a Pd centre. See paper: Bismillah, A. N.; Johnson, T. G.; Hussein, B. A.; Turley, A. T.; Saha, P. K.; Wong, H. C.; Aguilar, J. A.; Yufit, D. S.; McGonigal, P. R. Nature Chem. 2023, DOI:10.1038/s41557-023-01156-7
Luminescent Molecules
Organic molecules that absorb and emit light are of interest for use in technologies such as organic light-emitting diodes and photovoltaics. We investigate how noncovalent bonding interactions impact on the optoelectronic properties of luminescent materials. Understanding these phenomena is an important step to explaining how individually optimised components operate when combined in devices, e.g., how the interactions between chromophores and host materials alter emission energies. We investigate overcrowded chromophores bearing multiple aromatic rings, which generally improves solid-state emission, but can also lead to unusual photochemical reactivity and photophysical processes.
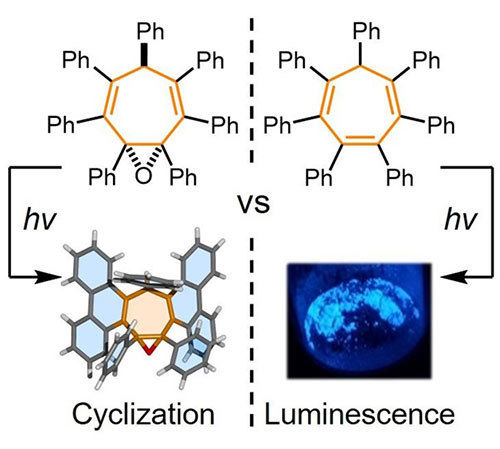
Perarylated cyclohetatriene chromophores show aggregation-induced emission properties. However, close structural analogues with decreased conjugation undergo rapid photocyclization rather than emission. See paper: Turley, A. T.; Saha, P. K.; Danos, A.; Bismillah, A. N.; Monkman, A. P.; Yufit, D. S.; Curchod, B. F. E.; Etherington, M. K.; McGonigal, P. R. Angew. Chem. Int. Ed. 2022, 61, e202202193
Aromatic Cation Materials
Using similarly overcrowded systems, we also investigate how molecular strain, charge and noncovalent bonding interactions impact upon the electronic structure of aromatic systems and their resulting properties. Aromatic rings are arguably the most important organic building blocks for materials ranging from drugs to plastics. We have found that balancing strain and aromatic stabilisation energy can create dynamic ring systems, which opens up new possibilities. Can the bonding in aromatic systems be made and broken on demand by the application of external stimuli?
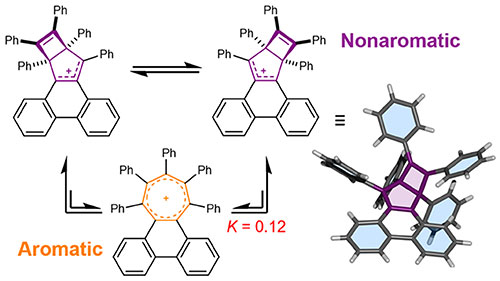
Introducing bulky groups around the periphery of a 6π aromatic cation (tropylium) causes strain, which destabilises the aromatic ring and places it in equilibrium with a nonaromatic isomer. See paper: Saha, P. K.; Mallick, A.; Turley, A. T.; Bismillah, A. N.; Danos, A.; Monkman, A. P.; Avestro, A.-J.; Yufit, D. S.; McGonigal, P. R. Nature Chem. 2023, DOI:10.1038/s41557-023-01149-6
Self-assembled Soft Surfaces
Friction between contacting surfaces reduces energy efficiency and causes irreversible damage, leading to an estimated 20% of global energy consumption. Natural systems minimise friction by exploiting surfaces that attract a lubricating layer of water molecules and replenishing surfaces as they become worn. We develop coatings that mimic these aspects of Nature’s lubricating surfaces. We use polymers that are designed to attract a layer of water molecules to create a slippery, low-friction surface. They coat the surface by taking advantage of reversible, yet selective, supramolecular binding interactions, which enable to surface to be easily repaired after they have been subjected to wear and tear.
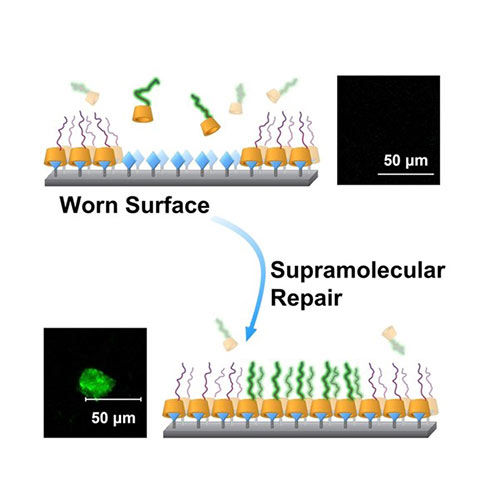
A graphical representation of the assembly of cyclodextrin-capped lubricating polymers on to an adamantane-finctionalised surface.. See paper: Wang, Y.; Sun, Y.; Avestro, A.-J.; McGonigal, P. R.; Zhang, H. Chem 2022, 8, 480–493

