Dr John Moore
01904 322548
Email: john.moore@york.ac.uk
Spectroscopy and Photochemistry in Solution
Our interests centre on the spectroscopy and photochemistry of molecules in solution, including the use of pulsed lasers to observe excited states and reactive intermediates in real time.
Our research has included studies of photoisomerisation, photodissociation, electron-transfer, energy-transfer, and photophysical mechanisms, and the observation of excited states, radicals, and other intermediates in organic, inorganic, and biochemical systems, as well as the development of laser techniques.
We have worked extensively with external collaborators. These links have included extended industrial collaborations with Unilever, DuPont, Zeneca, Avecia and Fujifilm, and also work at the UK Central Laser Facility within the Rutherford Appleton Laboratory, and at the European Laboratory for Non-Linear Spectroscopy in Florence.
Our interests include
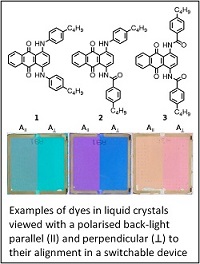
Liquid crystals and dyes
Dyes can be added to liquid crystals to produce guest-host materials, which can be used to produce colour display and other devices. The design of dyes that can meet the stringent criteria for such applications is challenging, and includes the need to produce strongly absorbing and stable dyes that are highly aligned within liquid crystal hosts, which themselves need to have high molecular alignments. We study the spectroscopy, reactivity and design of dyes, liquid crystals and guest-host mixtures, including experimental research that extends to devices, and a range of computational approaches. The general aim is to develop an understanding of the fundamental physical chemistry that defines the molecular and optical behaviour, and which can assist in the rational design of new materials for a variety of applications. Our research is carried out in close collaboration with the Liquid Crystal Group at York.
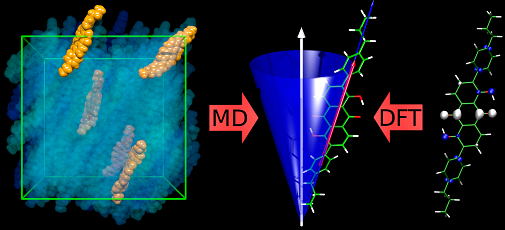
Photochemistry of organic dyes
Dyes and inks need to retain their colour when they are exposed to ambient room-light and sun-light. Unfortunately, many of the dyes which give the brightest colours also fade relatively quickly, and this photofading often occurs much more quickly when the dyes are deposited onto molecular surfaces such as cellulose, as found in paper and cotton. This problem can be particularly acute for dyes used in areas of new technology, such as ink-jet printing.
Our research focuses on two general areas, and includes studies of azo and xanthene dyes. We study how these dyes interact with molecular surfaces, such as cellulose, by applying a range of steady-state spectroscopic techniques to explore how the structures of the dyes are affected by their solvent and surface environments. We then use laser excitation and time-resolved laser techniques to study the photochemical reaction mechanisms of these dyes, both in solution and on surfaces.
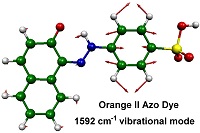
The techniques we use include UV-visible absorption and emission, IR absorption, resonance Raman, NMR, and ESR spectroscopy, allied with computational modelling and detailed data analysis. We use a wide range of lasers, including continuous-wave lasers for steady-state studies, and nanosecond and pico/femtosecond lasers for time-resolved UV-vis absorption, emission, IR, resonance Raman, and temperature-jump studies.
Light-controlled ion switches
Ion signalling is ubiquitous in nature. Calcium signalling, especially, is used to regulate many processes, including muscle contraction and neuron activity. We have been developing and studying "artificial" ion-signalling systems: our interests are in light-controlled ion switches which complex metal cations in solution and then release them when triggered to do so by light.
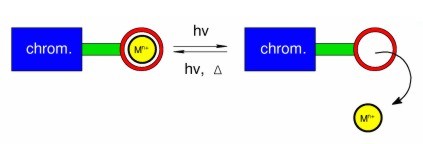
Our research has included all-organic systems in which ion-switching is based on photoisomerisation, and it has extended to inorganic systems, where photoinduced electron-transfer and energy-transfer mechanisms can also be important. We use a range of steady-state spectroscopic techniques to study these molecules and their complexes with metal cations in the ground state. We then use time-resolved laser techniques to study their excited states, in some cases enabling us to observe complete ion release-and-recapture cycles in real time.