Professor Richard Douthwaite
01904 324183
Email: richard.douthwaite@york.ac.uk
Molecular and materials chemistry, photocatalysis
Our research is currently focused on the synthesis, understanding and application of materials and metal complexes for catalysis using renewable energy.
Current main lines of research are outlined below but please visit the Group Pages for more information.
Photocatalysis and Solar Energy Conversion
Photocatalysts are a class of material that mediate chemical reactions using photons as a source of energy. There are many potential uses including the degradation of chemicals detrimental to the environment, clean chemical synthesis, and the conversion of solar into chemical energy (e.g. water to dihydrogen and dioxygen).
In principle, the concept is simple: to generate reducing (electrons) and oxidizing (holes) from excitation across a bandgap (Fig. 1), which can subsequently be used for redox chemistry. However, in practice there are tremendous challenges to address across efficiency, stability, cost, and practical implementation. It is unlikely a single compound will be able to perform all the required steps efficiently (absorption across the visible spectrum, electron/hole migration to the surface, and catalysis of the chosen reaction, whilst remaining stable for a long time). Therefore 'engineered' composites are the more likely solution where more than one material can be used to perform one or two of the underpinning functions.
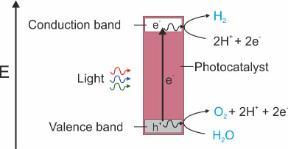
Figure 1
Examples from our research include macroporous TaON (Fig. 2a) and photocatalytic nanoparticles embedded in macroporous hosts (Fig. 2b) both for hydrogen production from water. Macroporous materials have interesting optical properties that can be exploited to increase the efficiency of a photocatalytic reaction. We have also studied metal oxides such as BiVO4 for selective oxidation of alcohols to aldehydes using oxygen as the chemical oxidant (Fig. 2c) avoiding the need for stoichiometric oxidants such as peroxides, manganate or chromate which require energy for synthesis or generate toxic waste.
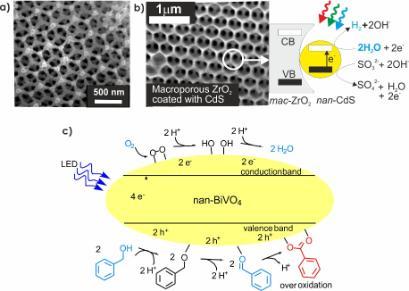
Figure 2
Photocatalytic concepts are also relevant to photoelectrochemistry where an electrical field (bias) can be used to increase electron-hole separation and the rate of redox catalysis. This technology also supports easier product separation. We use photoelectrochemistry to study photocatalytic materials and as the basis for simple photoelectrochemical devices. Examples include 3-dimensional transparent conducting oxides such as aluminium zinc oxide spheres (Fig. 3a) which can be coated with photoactive materials for hydrogen production, and porous BiVO4 for C-C bond formation such as the Kolbe reaction (Fig. 3b). These 3-D structures allow more light to be absorbed for a given surface area, and support greater electrode-electrolyte interfacial contact compared to planar electrodes.
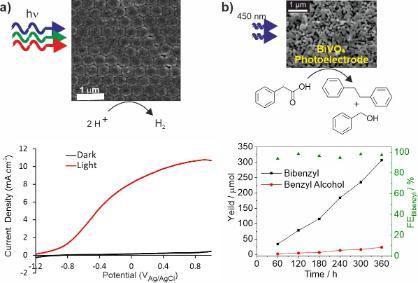
Figure 3
(Photo)catalysis using metal complexes
Metal complex catalysis has recently focused on synthesis and photocatalysis of Mo complexes for oxygen atom transfer and supporting Ir hydrogenation catalysts on carbon nitride (C3N4) substrates (Fig. 4). The aim here is to study structure property relationships to guide increases in activity and selectivity. For example, the Ir-C3N4 system contains Ir complexes coordinated to the edge sites of C3N4 planes which supports regioselective hydrogenation.

Figure 4
Environmental Transmission Electron Microscopy
The York JEOL Nanocentre commissioned (2024) a double aberration-corrected E(S)TEM facility with a bespoke differentially-pumped open cell for structural and spectroscopic analysis at high spatial and time resolution under gaseous atmospheres. The combination of simultaneous atomic scale imaging and energy dispersive X-ray (EDX) and electron energy loss spectroscopy (EELS) as well as 4D diffraction, with controlled electron dosing optimally allows simultaneous chemical composition and structure to be measured on the ms timescale in gaseous atmospheres. The combination of direct imaging, complemented by spectroscopic data optimally allows the generation of movies charting the evolution of structure and chemical properties such as oxidation state at the atomic scale.
Equipment
The Department of Chemistry has modern analytical facilities including NMR spectrometers (700, 600, 500, 400, 300, 270 MHz), IR, UV and ESR, fluorescence and time-resolved photoluminescence spectrometers; mass (ESI, FAB) spectrometers; powder diffraction, XRF, TGA, DSC, and BET surface area measurements. Through the York Jeol Nanocentre the group has access to an array of microscopy facilities including SEM, (S)TEM, and AFM.
In addition to standard laboratory equipment the group also has its own fibre optic UV-Vis and diffuse reflectance spectrometer, semi-prep HPLC, GC, autoclaves, carousel reactors, drybox, light sources (Xe-arc lamps and LED arrays with a monochromator for wavelength dependent measurements); a range of electrochemical workstations including Parstat 3000 and Biologic SP-200; film fabrication equipment including electrospray, spin coater, dip-coater, ozone cleaner; and contact angle measurement.

