Professor Anne-Kathrin Duhme-Klair
+ 44 (0)1904 322587
Email: anne.duhme-klair@york.ac.uk
Research Interests: Biological Inorganic Chemistry
Bioessential metal cations, such as Fe(III) and Mo(VI), play a key role in biological processes, such as electron transfer, oxygen binding and catalysis. We are interested in the strategies used by nature to bind these essential metal ions and to tune their reactivity. Once these strategies are understood, they can be applied to other areas of Biological Inorganic Chemistry. Current projects include:
1. New Insights into Siderophore-mediated Iron Uptake
Microorganisms produce and secrete siderophores to solubilise essential iron for uptake into the cell. We are interested in the chemical design features that allow siderophores of the 2, 3-dihydroxybenzamide type (Fig. 1) to bind Fe(III) and other essential metal ions, such as Mo(VI). The importance of the denticity, chirality and backbone structure of these chelators and the interactions with their cognate binding proteins are the focus of our research.
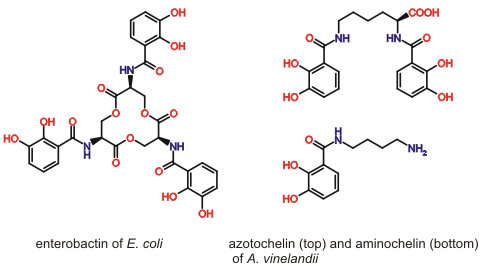
Figure 1. Selected 2, 3-dihydroxybenzamide siderophores.
By studying the interactions of periplasmic siderophore binding proteins, such as CeuE of the food-borne pathogen Campylobacter jejuni, with Fe(III) complexes of both hexadentate and tetradentate siderophores, we are identifying and exploiting the structural changes that allow this bacterial protein to capture more than one type of ferric siderophore (Fig. 2, collaboration with Professor K S Wilson).
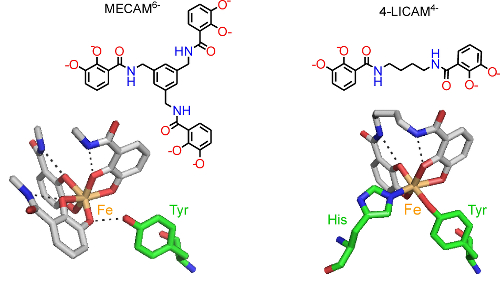
Figure 2. Fe(III) (orange) coordinated to the siderophore mimics MECAM6- and 4-LICAM4- (coloured by atom type) with key amino acid residues in the binding pocket of CeuE shown (green cylinders as carbon atoms).
2. The Development of Artificial Metalloenzymes
Artificial metalloenzymes can combine the beneficial features of both, reactive synthetic catalysts and highly selective protein scaffolds. In this way, synthetic reactions can be performed with new levels of selectivity and in a biocompatible environment. Inspired by the way bacteria use siderophores for the uptake of essential iron, we are using siderophores as new redox-reversible anchoring groups for synthetic catalysts. In the presence of Fe(III) siderophore-based anchors strongly connect organometallic catalysts to protein scaffolds, creating artificial metalloenzymes, but on reduction to Fe(II), dissociation takes place and the artificial enzyme disassembles (Fig. 3, collaboration with Prof. K. S. Wilson). This reversible anchoring approach allows high value components, in particular the protein, to be reclaimed and reused.
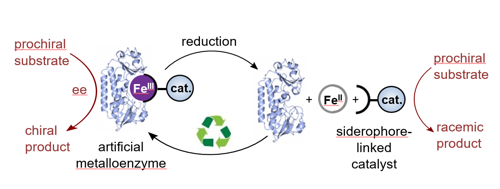
Figure 3. A redox reversible iron-siderophore anchor connects a synthetic catalyst to a protein scaffold, thereby allowing the controlled assembly and disassembly of an artificial metalloenzyme.
3. The Development of New Antimicrobials
Due to an increase in bacterial resistance to antibiotics, there is an urgent need for novel ways of combating bacterial infections. By linking antimicrobial agents to Fe(III) siderophore complexes that bacterial cells needs to survive, we aim to promote the active uptake of the antimicrobial and overcome resistance arising from changes to cell permeability and drug efflux (Trojan horse approach). Current work exploits a number of siderophores in combination with fluoroquinolone antimicrobials (Fig. 4, collaboration with Dr A Routledge and Professor G H Thomas).
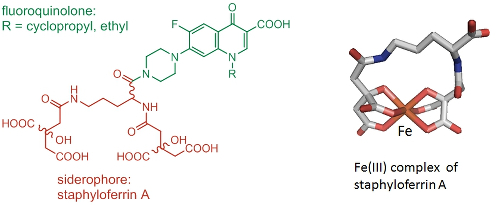
Figure 4. Trojan horse antimicrobial agent consisting of staphyloferrin A and a fluoroquinolone (left). Structure of the Fe(III) complex of (S),(R),(R)-staphyloferrin A (right).
4. Bioinspired oxygen atom transfer with light
Nature uses molybdenum-containing enzymes to catalyse oxygen atom transfer (OAT) from water to organic substrates. In these enzymes, the two electrons that are released are removed by spatially-separated electron transfer units. We are particularly interested in the importance of these spatially-separated one-electron transfer units and the participation of non-innocent ligands in OAT catalysis. Inspired by the molybdoenzymes, we are developing Mo(VI) complexes that allow the rate-determining step, the transfer of an oxo ligand to PPh3, to be photoactivated (Fig. 5, collaboration with Professor R N Perutz).
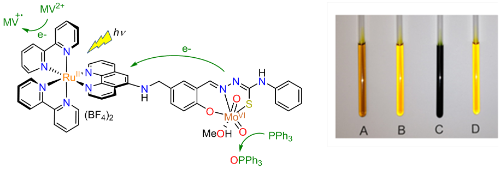
Figure 5. Structure of the dyad [Ru(bpy)2(L)MoO2(MeOH)](BF4)2 (left) and photographs taken of samples after irradiation (right). Sample A: [Ru(bpy)2(L)MoO2(solv)]2+, PPh3 and methyl viologen (MV2+); sample B: control kept in the dark; sample C: [Ru(bpy)2(H2-L)]2+, PPh3 and MV2+; sample D: [Ru(bpy)2(L)MoO2(solv)]2+, PPh3 but no MV2+.

