Professor Victor Chechik
01904 324185
E-mail: victor.chechik@york.ac.uk
Research
Supramolecular/nanoscale chemistry, mechanisms of radical reactions and EPR spectroscopy
The Chechik group uses mechanistic understanding to solve a range of topical problems including interactions and reactions in supramolecular systems and in nanomaterials. We often use stable free radicals and EPR spectroscopy as probes for these systems (we have three CW-EPR spectrometers operating at X- and Q-bands). We are also interested in free radical chemistry in general including detection and characterization of short-lived radical intermediates and mechanisms of radical reactions. We often collaborate with other researchers in academic and in industry. Current projects include the following areas:
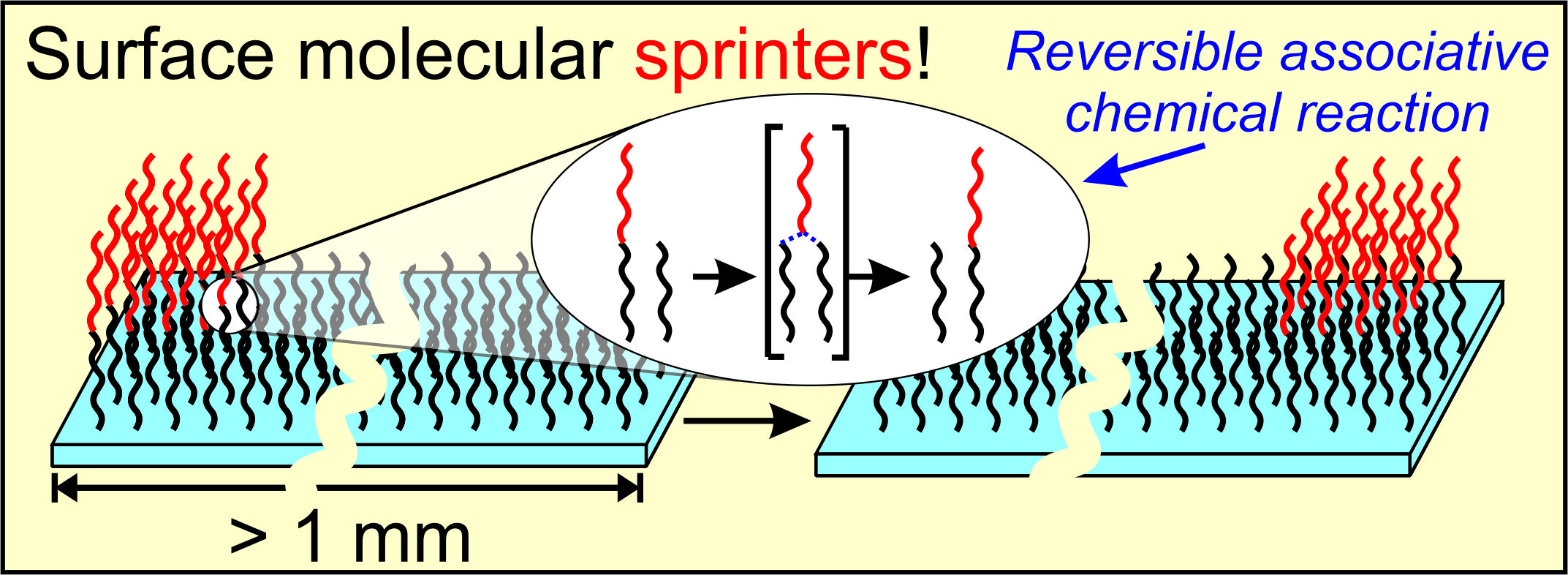
Surface Molecular Walkers
We aim to design molecules that can rapidly move on the surfaces. We target the speed of movement that will give macroscopic displacement (millimeters) in a reasonable time (hours). Our molecular walkers are based on reversible and associative chemical reactions on the surface of a reactive organic monolayer deposited on a suitable solid support.
 |
|---|
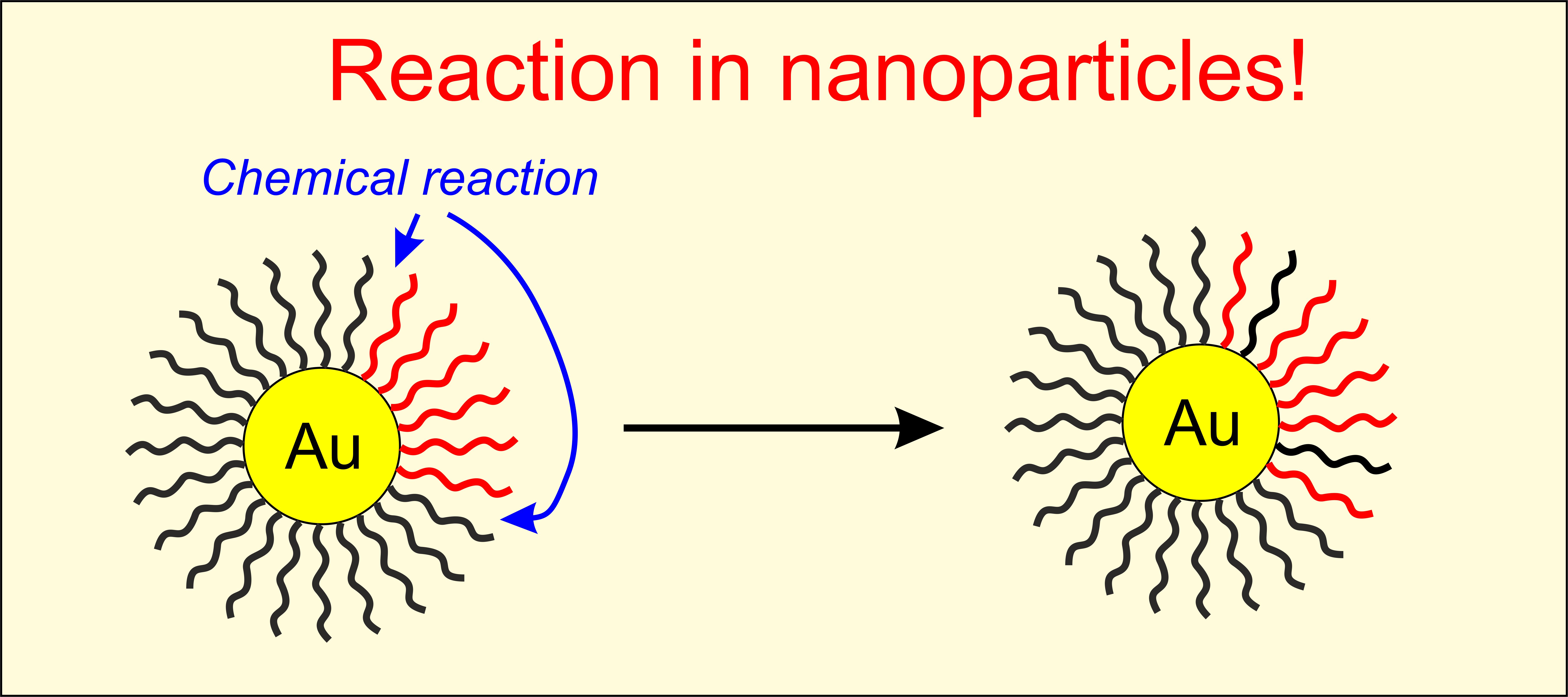
Molecular interactions in nanoparticles
We have long been interested in probing mobility, reactivity and non-covalent interactions of organic molecules deposited on the surface of inorganic nanoparticles. We often use TEMPO derivatives and EPR spectroscopy to monitor and understand these systems. For instance, one current project explores reactions between neighbouring molecules on the nanoparticle surface. Functional nanomaterials have many important applications and understanding how they can be manipulated through chemical reactions is essential for their future development.
 |
|---|
Magnetic nanoparticles for therapeutic delivery
Magnetic nanoparticles (eg with an iron oxide core) bring together many properties of supramolecular systems. They can be easily functionalised with organic ligands that determine their solubility and other properties, several functional ligands can be attached to the same nanoparticle, they can be guided by static magnetic field and heated by oscillating magnetic field. We are interested in using these materials for medicinal applications. In particular, we work on developing functional organic coatings for nanoparticles that would provide active targeting and delivery of therapeutic agents.
  |
|---|
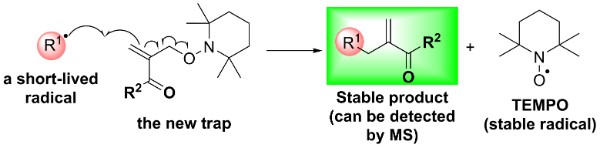
Free radical reactions and radical intermediates
Short-lived free radical intermediates play a key role in many important processes, including combustion, polymerisation, atmospheric reactions, catalysis and biological process. However, they are difficult to study as they are usually present at extremely low concentrations. We are interested in developing methodologies for detecting short-lived radicals. We have recently developed a new type of radical traps for detection with mass spectrometry. We are using these traps (as well as more conventional spin trapping approach and EPR spectroscopy to understand the mechanisms of radical reactions. One current project aims to quantitatively measure radical concentrations – a really challenging task! We are also interested in understanding radical reaction mechanisms in complex biologically-relevant systems.
 |
|---|
Group members
Dr Andrew West – Postdoctoral Research Associate
Andrew joined the group in 2024 as a postdoc to work on the development of novel and fast small-molecule molecular walkers. He originally completed his PhD in Edinburgh working with Professor Cockroft, investigating various forms of halogen bonding in supramolecular systems. After this he worked with Professor Gothelf in Aarhus working on ssDNA rotaxanes and novel DNA triplex bases. Outside of academic pursuits, Andrew enjoys bouldering and running as well as learning about history.
 |
|---|
Amber Covington – PhD student
Amber completed her MSci in Physics and Chemistry from Durham University in 2024, with her Masters project focusing on using structured ternary fluids to form cocrystals. She started her PhD the same year, researching molecular walkers based on electron self-exchange with Professor Victor Chechik. In her spare time, she enjoys aikido, crochet, sewing and art.
 |
|---|
Edward Jijie – PhD student
Edward completed his MSci in Chemistry from Imperial College London in 2024. In the summers of 2022 and 2023 he was a research intern in Prof. Phil Parsons’ group working on reaction methodology of radical ring expansion of thietane-3-ol derivatives. His Masters’ thesis focused on spectroelectrochemical studies of the pathogenic enzyme MsrQ, under the supervision of Dr. Maxie Roessler. In 2024 he joined Prof. Victor Chechik’s group as a PhD student, working on mechanistic studies of radical reactions in soft materials and hair damage. This project is funded by the Department of Chemistry at the University of York and by an industry collaborator, Procter & Gamble. In his free time, he plays for the University Table Tennis team and enjoys hiking on the occasional sunny days, cooking and swimming.
 |
|---|
Ben Woodward – PhD student
Ben completed his integrated Master’s at Durham University with his final year in Germany at JMU Würzburg focusing on the synthesis and spectroscopic properties of electron donor-acceptor dyads. He started his PhD in the Chechik group in 2024. His project aims to use SH2’ radical traps to quantify radical intermediates in organic reactions. This will be achieved by using isotopically labelled traps, enabling quantification by mass spectrometry. Ultimately this means kinetic models can be built, leading to greater mechanistic understanding. Outside the lab, he enjoys sport and is a member of the University triathlon club.
 |
|---|
Publications
Selected recent publications:
- Iqbal, A. Wolstenholme-Hogg, A. R. Gompels, V. Chechik, Quantitative Characterization of Organosilane Monolayers by Oxidative Dissociation of Monolayer Molecules, Anal. Chem. 2025, 97 (8), 4661–4667.
- Sharma, D. Ungar, E. Dyson, S. Rimmer, V. Chechik, Functional Magnetic Nanoparticles for Protein Delivery Applications: Understanding Protein-Nanoparticle interactions, Nanoscale 2024, 24 (5), 2466-2477.
- Vagkidis, L. Li, J. M. Marsh, V. Chechik, Synergy of UV light and heat in peptide degradation, J. Photochem. Photobiol. A, 2023, 439, 114627.
- J. H. Williams, G. A. Boustead, D. E. Heard, P. W. Seakins, A. R. Rickard, V. Chechik, New Approach to the Detection of Short-Lived Radical Intermediates, J. Am. Chem. Soc. 2022, 144 (35), 15969-15976.
- J. Greener, P. Ubysz, W. Owens-Ward, G. Smith, I. Ocana, A. C. Whitwood, V. Chechik, M. J. James, Radical-anion coupling through reagent design: hydroxylation of aryl halides, Chem. Sci. 2021, 12 (43), 14641-14646.
- D. Nicholas, V. Chechik, Characterization of Host–Guest Complexes of Supramolecular Self-Assembled Cages Using EPR Spectroscopy, J. Phys. Chem. B 2020, 124 (27), 5646-5653.
You can find Victor’s full publication list in York Research Database.
Alumni
This tab is under development. Please check our old web page to learn about previous group members.
Photos
This tab is under development. Please check our previous web page for old photos.

