Professor Gideon Davies, FMedSci, FRS
Royal Society Ken Murray Research Professor
Email: gideon.davies@york.ac.uk
Profile
Professor Gideon J. Davies FMedSci, FRS leads a research programme at the interface of Chemistry and Biology, focused on the structural enzymology and chemical biology of carbohydrate-active enzymes. His group studies proteins involved in the synthesis, modification, and degradation of complex glycans, combining structural, mechanistic, and application of chemistry approaches. Stimulated by the organic chemistry of enzymes, the team develops and applies technologies — including activity-based probes and mechanism-based inhibitors — to understand glycan function in health, disease, and the environment. Their work has wide-reaching applications, notably in biotechnology and microbial ecology but also in antiviral, anti-neurodegeneration and anticancer therapeutics.
In the Department of Chemistry, Gideon’s teaching includes the Year 3 Chemical Biology Core course in Frontiers of Chemistry 1 and the Building a Biological Background course in Year 1.
On-going research projects are outlined on the “Research” tab with representative Publications on the Publications Tab. Some recent/important reviews are given below:
Recent Reviews
Pickles, I.B., et al., (2025) Activity-based probes for dynamic characterisation of polysaccharide-degrading enzymes. Biochemical Journal, 482, 939-954. https://doi.org/10.1042/
Artola, M., et al., (2024) From mechanism-based retaining glycosidase inhibitors to activity-based glycosidase profiling. J Am Chem Soc. 146, 24729–24741. https://doi.org/10.1021/jacs.4c08840
Walton, P. H., et al., (2023) The Histidine Brace: Nature’s Copper Alternative to Haem? FEBS Lett. 597, 485-494. https://doi.org/10.1002/1873-3468.14579
McGregor, N. G. S et al., (2022) Detecting and Identifying Glycoside Hydrolases Using Cyclophellitol-Derived Activity-Based Probes. Methods Enzymol. 664, 103-134. https://doi.org/10.1016/bs.mie.2022.01.007
Snow, A. J. D., et al., (2021) Sulfoglycolysis: catabolic pathways for metabolism of sulfoquinovose. Chem Soc Reviews. 50, 13628-13645. https://doi.org/10.1039/D1CS00846C
Rovira, C. et al., (2020) Mannosidase mechanism: At the intersection of conformation and catalysis. Curr Opin Struct Biol. 62, 79-92. https://doi.org/10.1016/j.sbi.2019.11.008
King, D., et al., (2019) Molecular mechanisms regulating O-GlcNAc processing enzymes. Curr Opin Chem Biol. 53, 131-144. https://doi.org/10.1016/j.cbpa.2019.09.001
Ciano, L., et al., (2018) Bracing copper for the catalytic oxidation of C–H bonds. Nature Catalysis. 1, 571-577. https://doi.org/10.1038/s41929-018-0110-9
Davies, G.J et al., (2012) Conformational analyses of the reaction coordinate of glycosidases. Acc Chem Res 45, 308-316. https://doi.org/10.1021/ar2001765
Research
Research
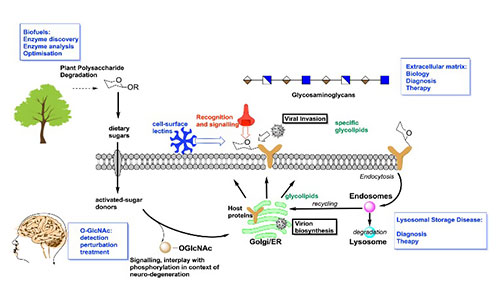
Our research covers a wide range of areas, all linked by the common theme of 3-D structure, reaction mechanism and chemical biology in glycoscience / carbohydrate enzymology. The work has many societal ramifications in biotechnology, microbiotal health, and drug design for lysosomal storage diseases (Gaucher, Pompe, Fabry etc), cancer (anti-heparanase compounds), anti-virus (anti glucosidase I and II compounds, neuraminidase inhibitors) and neurodegeneration (O-GlcNAc).
Early Career
Davies made foundational contributions to biotechnology by solving the 3D structures, ligand binding and catalytic mechanism of numerous carbohydrate-active enzymes (CAZy families), many of which are central to industrial processes. Beginning with cellulases (Nature, 1993), his work expanded to key enzymes such as α/β-mannanases, α/β-glucosidases, xylanases, arabinofuranosidases, xyloglucanases, and pectate lyases—along with their substrate-binding carbohydrate-binding modules (CBMs).
In a landmark collaboration with Withers, Davies redefined the catalytic mechanism of lysozyme by capturing a covalent intermediate (Nature, 2001), challenging textbook views. Later, with Gilbert (Newcastle) and Brumer (UBC), he helped uncover how enzyme consortia act synergistically to break down complex plant polysaccharides like xyloglucans, mannans, and rhamnogalacturonans—insights published in Nature (2014, 2015, 2017) that have had wide-reaching implications for bioenergy, agriculture, and sustainable materials.
- Ndeh, et al., 2017. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature https://doi.org/10.1038/nature21725
- Cuskin, et al., 2015. Human gut Bacteroidetes can utilise yeast mannan through a selfish mechanism. Nature https://doi.org/10.1038/nature13995
- Larsbrink, et al., 2014. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature https://doi.org/10.1038/nature12907
- Vocadlo, et al., 2001. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature https://doi.org/10.1038/35090602
Much of the group's work has focussed on the chemical and mechanistic aspects that 3D fold alone cannot define – the reaction coordinate and substrate distortion.
Some recent areas of research are outlined below:
Activity-Based Probes
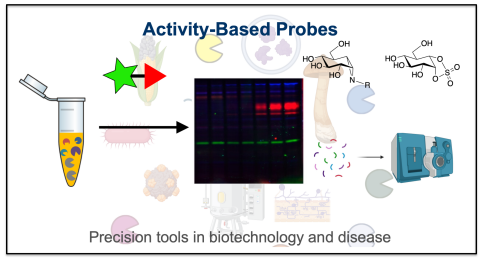
Funded through an ERC Synergy grant with Hermen Overkleeft in Leiden and Carme Rovira in Barcelona we have an extensive project on the design and application of precision chemical tools known as activity-based probes in both biotechnological and medical domains of application.
In biotechnology, these probes allow us to dynamically characterize enzymes involved in cellulose, xylan, starch and xyloglucan degradation and identify their function within microbial communities and the mammalian microbiota. For example, we recently introduced highly selective α-amylase probes that discriminate between linear and branched-chain starch-degrading enzymes, enabling functional annotation at an unprecedented resolution (Pickles et al., 2025). We also developed a multiplexing ABPP platform to dissect native bacterial xyloglucan-degrading systems (McGregor et al., 2023). We have applied these probes with Sarel Fleishmann for protein design and evolution strategies (Lipsh-Sokolik, R., et al. (2023)).
- Pickles, I. B., et al. (2025) Precision activity-based α-amylase probes for dissection and annotation of linear and branched-chain starch degrading enzymes. Angew Chemie Int Ed. e202415219https://doi.org/10.1002/anie.202415219
- McGregor, N. G. S., et al. (2023) A multiplexing activity-based protein profiling platform for dissection of a native bacterial xyloglucan-degrading system. ACS Central Science. 9, 2306–2314. https://doi.org/10.1021/acscentsci.3c00831
- Lipsh-Sokolik, R., et al. (2023) Modularly designed protein fragments combine into thousands of active and structurally diverse enzymes. Science. 379, 195-201. https://doi.org/10.1126/science.ade943
In biomedicine and cancer our structural enzymology has enabled us to design mechanism-based inhibitors with therapeutic promise. Our teams developed Epi-cyclophellitol cyclosulfate, a potent ER α-glucosidase II inhibitor that blocks replication of SARS-CoV-2 and related coronaviruses (Thaler et al., 2024). In oncology, we created novel heparanase inhibitors that reduce cancer metastasis in vivo (de Boer et al., 2022), paving the way for new anti-metastatic therapies.
- Thaler, M., et al. (2024) Epi-cyclophellitol cyclosulfate, a mechanism-based ER α-glucosidase II inhibitor, blocks replication of SARS-CoV-2 and other coronaviruses. ACS Central Science. 10, 1594–1608. https://doi.org/10.1021/acscentsci.4c00506
- de Boer, C., et al. (2022) Mechanism based heparanase inhibitors reduce cancer metastasis in vivo. Proc Natl Acad Sci USA. 119, e2203167119. https://doi.org/10.1073/pnas.2203167119
Sulfoglycolysis

Sulfoglycolysis, the bacterial metabolism of the sulfosugar sulfoquinovose, plays a crucial role in the global sulfur cycle and thus agricultural biotechnology. In a long-standing collaboration with Spencer Williams and Ethan Goddard-Border in Melbourne, led by senior PDRA Mahima Sharma in York, our team has elucidated the molecular basis for sulfosugar recognition and selectivity (for example, (Sharma et al., 2021)). Recently we discovered and characterised a new NAD+ dependent enzyme involved in sulfoquinovose degradation, allowing the identification of many new pathways (Kaur et al., 2024). These insights are advancing our understanding of sulfur biogeochemistry and microbial ecology. This work led to the Royal Society of Chemistry Horizon Team Prize for work on sulfoquinovose, in 2024.
- Sharma, M., et al. (2021) The Molecular Basis of Sulfosugar Selectivity in Sulfoglycolysis. ACS Central Science. 7, 476–487. https://doi.org/10.1021/acscentsci.0c01285
- Kaur, et al., 2023. A widespread family of oxidoreductive sulfoquinovosidases at the gateway to sulfoquinovose catabolism. J Am Chem Soc, 146, 125-133 https://doi.org/10.1021/jacs.3c11126
The O-GlcNAc Modification
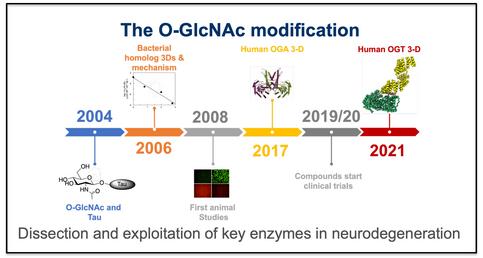
The group has a long-standing interest in the O-GlcNAc modification in human cells and its link to neurodegeneration. Recent work has involved the study of the full-length O-GlcNAc transferase (OGT) by CryoEM, illuminating the dimeric arrangement of human OGT and enhancing our mechanistic understanding of substrate recognition (Meek et al., 2021). Our body of work on the O-GlcNAc hydrolase provides the structural; and mechanistic framework for drug discovery targeting this post-translational modification which is central to numerous neurodegenerative diseases.
- Meek, R. W., et al., (2021) Cryo-EM provides insights into the dimer arrangement of OGT. Nature Commun. 12, Article number: 6508. https://doi.org/10.1038/s41467-021-26796-6
(Lytic) Polysaccharide Monooxygenases ([L]PMOs)
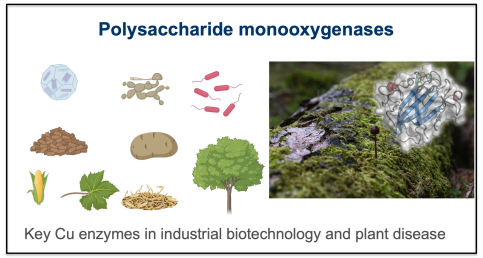
In a long-standing collaboration with Paul Walton we have discovered and dissected many polysaccharide monooxygenases involved in biotechnology and more latterly in plant infection (for example, with Fede Sabbadin in Biology, how secreted pectin monooxygenases facilitate plant infection by pathogenic oomycetes (Sabbadin et al., 2021). This research thus intersects plant pathology, enzymology, and biorefinery science, and is guiding the development of enzyme cocktails for biomass conversion and plant disease control. The biotechnological aspects were rewarded with the iChemE Global Energy Award of the Institute of Chemical Engineers in 2016.
https://www.york.ac.uk/news-and-events/news/2016/quality/icheme-global-award/
- J. Quinlan, et al., 2011. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Natl Acad Sci USA, 108, 15079-15084. https://doi.org/10.1073/pnas.1105776108
- Sabbadin, F., et al. (2021) Secreted pectin monooxygenases drive plant infection by pathogenic oomycetes. Science. 373, 774-779. https://doi.org/10.1126/science.abj1342
Glycosyltransferase Structure and Mechanism
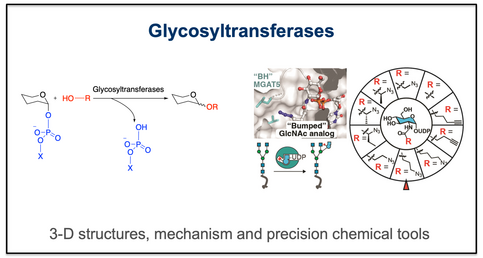
The group has a very long-standing interest in glycosyltransferases – the enzymes that catalyse the synthesis of glycosidic bonds in glycans, olio and polysaccharides and glycoconjugates. Our structural and mechanistic studies of glycosyltransferases have illuminated how these enzymes orchestrate the formation of glycosidic bonds with exquisite specificity. His work has clarified the catalytic roles of donor and acceptor binding, metal ion coordination, and conformational change, revealing how subtle variations in active site architecture lead to diverse sugar linkages. This mechanistic understanding has enabled advances in synthetic biology, vaccine design, and enzyme engineering. In addition to our work on the O-GlcNAc transferase, ongoing projects include human MGATV, which plays a key role in cancer.
- Darby, et al., 2020. Substrate engagement and catalytic mechanisms of N-acetylglucosaminyltransferase V. ACS Catalysis, 10, 8590–8596 https://doi.org/1021/acscatal.0c02222
- Liu, et al., 2024. A Bioorthogonal Precision Tool for human N-acetylglucosaminyltransferase V. J Am Chem Soc, 146, 26707–26718 https://doi.org/10.1021/jacs.4c05955
Biography
Biography
Gideon Davies received his PhD from the University of Bristol in 1990 and went on to postdoctoral research at EMBL Hamburg and CNRS Grenoble with periods at the University of Uppsala with Alwyn Jones, FRS and with Steve Withers,FRS as the inaugural “Peter Wall Catalytic Visitor” at the University of British Columbia.
He moved to YSBL in York as a Postdoctoral worker with Guy Dodson, FRS before obtaining, in 1996, a Royal Society University Research Fellowship to work on Carbohydrate-Active Enzymes. He was made an Anniversary Professor of the University of York in 2001.
Gideon is most renowned for his work on carbohydrate-active enzymes, their 3D structures, reaction mechanisms and conformational itineraries, though to in vivo dissections and societal applications notably in biotechnology and also drug development.
Gideon was elected a Fellow of The Royal Society in 2010, and as a member of the European Molecular Biology Organization the same year and elected a Fellow of the Academy of Medical Sciences in 2014.
Amongst career highlights, has won many awards, recent prizes include the 2024 Centenary Award of The Biochemical Society, and the 2024 Chemistry Biology Interface Royal Society of Chemistry Horizon Prize.
Gideon Davies was made the Royal Society Ken Murray Research Professor in 2016.
He currently sits on the Council of the Royal Society.

Photograph of Gideon Davies taken upon the renewal of his Royal Society Research Professorship.
Group
Current Group Members

Dr Thamy Corrêa. Thamy is a Postdoctoral Research Associate in Chemical Biology with focus on Glycoscience, working with Prof. Gideon J. Davies, FMedSci, FRS, at the York Structural Biology Laboratory. She completed her PhD in Microbiology from the University of Viçosa, Brazil, followed by research positions in academia, a national laboratory and industry. Enthusiastic about the biochemistry and catalytic mechanistic of metalloenzymes, glycoside hydrolases, and esterases, her current research focuses on 1, enzymes involved in or facilitating the degradation of synthetic polymers; and 2, the development and application of molecular tools to probe glycoprocessing enzymes relevant to human health and green chemistry. Outside the lab, Thamy enjoys functional training, photography and watercolour painting.
https://www.york.ac.uk/chemistry/research/ysbl/people/staff/thamy-correa/
Dr Isabelle Pickles. Isabelle is a Postdoctoral Research Associate, studying carbohydrate active enzymes using chemical and structural biology. She uses activity-based probes to study biomass degradation in the context of both industrial starch processing and complex carbohydrate digestion in the human gut. In 2017, Isabelle graduated from Durham University with an MChem, before moving to the University of Leeds to pursue a PhD with Prof. Robin Bon. At Leeds she developed a panel of chemical tools to study TRPC1/4/5 ion channels, including photoaffinity probes and covalent inhibitors, graduating in 2022. Isabelle was one of the winners of the Royal Society of Chemistry Horizon Team Prize for work on sulfoquinovose, in 2024.
https://www.york.ac.uk/chemistry/research/ysbl/people/staff/isabelle-pickles/
Dr Olga Moroz joins the Davies group after a long and successful in YSBL, latterly with Keith Wilson on the Novozymes collaboration. During this time, she analysed hundreds of protein structures of key enzymes in industrial biotechnology and enzymes for societal use. Recent examples include Dispersins and other biofilm-active enzymes. She is currently performing both CryEM and X-ray analyses on diverse glycosidases and glycosyltransferases.
https://www.york.ac.uk/chemistry/research/ysbl/people/staff/omoroz/
Dr Mahima Sharma is a senior Research Fellow with expertise in structural enzymology and biocatalysis. She is currently working as a RCo-Investigator on BBSRC grant BB/W003805 on the molecular dissection of sulfoglycolysis pathways.
Mahima was one of the winners of the Royal Society of Chemistry Horizon Team Prize for work on sulfoquinovose, in 2024 making it a double for her having first won this prize in 2021 for her work with Professor Gideon Grogan
https://www.york.ac.uk/chemistry/research/ysbl/people/staff/msharma/
Wendy Offen. Works on diverse carbohydrate active enzymes. She is often involved in project inception, initial cloning and expression and in many crystal structure analysis of enzymes and their ligand complexes.
https://www.york.ac.uk/chemistry/research/ysbl/people/staff/woffen/
Ruby Williams is a PhD student on the ERC activity-based probe project (with Leiden and Barcelona) working on inhibitors of human glucosidase I and II.
Sophie Reynolds is a PhD student shared with Prof Paul Walton working on Lytic polysaccharide monooxygenases.
Some Past Group Members and Their Successes
Former group members have gone on to positions across many disciplines. Notably in Academia (many in UK, France, Germany, Canada, Poland, etc), in Industry (UK, USA) and into teaching (UK, France). Some examples are given below:
Industry
Former PhD student Carlos Martinez Fleites is a Scientific Leader at Molecular Modalities Discovery within Research Technology at GSK Stevenage. In York he specialised in the structural biology of diverse carbohydrate active enzymes, contributing to the development of selective inhibitors for disease-related enzymes such as those working on O-GlcNAc and in bacterial LPS biosynthesis. https://www.linkedin.com/in/cmartinezfleites
Former PDRA Andrew Thompson is Principal Scientist in Antibody Engineering at Cell Signaling Technology, Massachusetts. His work currently centres on bioconjugation, developing new methods & technologies in protein glycosylation, protein-protein interactions, and enzyme evolution. During his time in York, he focussed on the structural and functional analysis of protein-carbohydrate interactions, aiming to elucidate receptor/substrate binding modes and reaction coordinates; notably on diverse mannsoidases and α–mannanases.
Recent PhD graduate Elisha Moran is a biophysical characterisation scientist working in antibody therapeutic developability at LifeArc Scientific. In York she worked on the CryoEM analysis of neuraminidase, heparanase inhibitors for cancer and anti SARS-Cov2 agents. https://www.lifearc.org/
Former PhD student Rhianna Rowland is a Senior Structural Biologist in the Structural and Biophysical Sciences team within the Target Discovery division at GSK (Stevenage). After leaving York, she was a postdoctoral research associate in the Endicott & Noble structural biology group at the CRUK Newcastle Cancer Centre using single particle cryo-EM for the structural elucidation of protein complexes involved in cell cycle regulation and transcriptional events to support structure-based drug discovery. In York she worked on the human enzymes GBA1 and GBA2 and their link to generic diseases.
Dr Megan Bennett is now a Developmental Protein Biochemist at Oxford Nanopore Technologies, working on the engineering and development of the biological components of their sequencing platform and technology. In YSBL she worked on the structural and biochemical characterisation of a range of glycoside hydrolases and transferases using mainly X-ray crystallography and enzyme kinetics.
Academia – UK
Tracey Gloster
Professor at the University of St Andrews; Winner of the Biochemical Society Early Career Award (2012)
Prof. Gloster's research delves into the structure and function of eukaryotic carbohydrate-processing enzymes, with implications for understanding diseases like cancer and neurodegenerative disorders.
St Andrews Profile | Wikipedia
Richard Meek
University of Southampton Anniversary Fellowship Holder
Dr. Meek investigates eukaryotic glycobiology, focusing on carbohydrate-active enzymes and post-translational modifications, contributing to insights into diseases such as Alzheimer's and Parkinson's.
Profile
Liang Wu
Wellcome Trust Sir Henry Dale Fellow, Rosalind Franklin Institute
Dr. Wu's team explores the role of enzymes in the production of heparan sulfates, utilizing advanced imaging to study these complex sugar chains present in all cells.
RFI Profile
Glyn Hemsworth
Associate Professor of Structural Biology, University of Leeds; Winner of Biochemical Society Early Career Award (2015)
Dr. Hemsworth's expertise lies in structural biology, focusing on enzymes involved in the oxidative degradation of biomass, with applications in biotechnology.
Leeds Profile
Ed Taylor
Associate Professor, University of Lincoln. Ed is the lead for Microbiology and Biotechnology at the University of Lincoln. His work includes carbohydrate-actives enzymes, and enzyme for biocatalysis and drug synthesis.
https://staff.lincoln.ac.uk/50b410f4-ccc8-43ec-a501-0acd6b9416e2
Academia – Canada
Professor Dave Vocadlo
Professor of Chemistry, Simon Fraser University
Prof. Vocadlo leads research in chemical glycobiology, developing tools to study carbohydrate roles in biology and pioneering glycosylation inhibitors for neurodegenerative diseases.
SFU Profile
Professor Dave Zechel
Professor at Queen's University
Dr. Zechel's research focuses on discovering and characterizing unusual enzyme reactions from microbial pathways, aiming to develop enzyme-based systems for bioremediation and drug synthesis.
Zechel Lab
Professor Al Boraston
Professor of Biochemistry and Microbiology, University of Victoria
Prof. Boraston's work centres on carbohydrate-protein interactions, studying mechanisms of carbohydrate recognition and their roles in host-pathogen interactions and biotechnology.
UVic Profile
Academia – Germany
Christian Roth
Project Leader at Max Planck Institute, Potsdam
Dr. Roth leads the Carbohydrates: Structure and Function group, focusing on the structural biology of carbohydrate-active enzymes and their applications in biotechnology.
MPIKG Profile
Academia – France
Gerlind Sulzenbacher
Joint Director, AFMB CNRS Marseille
Dr. Sulzenbacher specializes in structural glycobiology and neurobiology, contributing to the understanding of protein-carbohydrate interactions through crystallography.
AFMB Profile
Annabelle Varrot
CNRS Research Director, Structural and Molecular Glycobiology Team, CERMAV-CNRS
Dr. Varrot's research focuses on fungal lectins, exploring their potential as drug targets and biomolecular tools in structural and molecular glycobiology.
ORCID
Florence Vincent
Researcher, AFMB CNRS Marseille
Dr. Vincent investigates enzymes involved in bacterial cell wall lysis and biofilm development, contributing to understanding bacterial pathogenicity and resistance mechanisms.
AFMB Profile
Nicolas Tarboureich
Associate Professor, Université Grenoble Alpes
Dr. Tarboureich's work includes structural studies of viral proteins, contributing to insights into virus-host interactions and potential therapeutic targets.
ORCID
Didier Nurizzo
Principal Beamline Operation Manager, ESRF Grenoble
Dr. Nurizzo manages the MASSIF-1 beamline, specializing in X-ray diffraction and automation for macromolecular crystallography, facilitating structural biology research.
Academia – Poland
Lukasz Sobala Assistant Professor, Hirszfeld Institute, Polish Academy of Sciences
https://hirszfeld.pl/en/structure/laboratories/laboratory-of-glycobiology/
Science Education
Imogen Breen
Head of Science, UK School
Ms. Breen leads science education initiatives, fostering scientific curiosity and understanding among students.
Sabine Leydier
Head of Science, French School
Ms. Leydier oversees science curriculum development and instruction, promoting scientific literacy in the educational community.
Awards
Awards
Professor Davies has been fortunate enough to win several awards which reflect the dedication of our research team. These include
Royal Society "Ken Murray" Research Professor, 2016-2026
Elected Fellow of The Academy of Medical Science, 2014
Elected Fellow of The Royal Society, 2010
Elected Member of The European Molecular Biology Organization, 2010
|
Non-RSC awards (selected) |
Roy Soc Chem awards |
|
Queen’s Anniversary Prize (to YSBL), 2019 |
|
|
Haworth Memorial Award, 2018 |
|
|
Khorana Prize, 2014 |
|
|
Peptide and Protein Award, 2008 |
|
|
Corday-Morgan Medal, 2001 |
|
|
Carbohydrate Chemistry Award, 1998 |
In addition, he was the Landsdowne Award Lecture recipient for Public Understanding of Science at the University of Victoria and gave the Choh Hao Li Memorial lecture of the Academia Sinica, Taiwan.
News
Dec 2025
Signal-strapping published in Nature Communications
Metalloproteins are often discovered after the fact - only once a protein’s activity is shown to depend on a metal ion. In contrast, signal-strapping bootstraps discovery directly from sequence: Signal peptides are artificially appended with a N-terminal histidine (based on our past LPMO work) to generate probes for proteome searches, enriching for proteins predicted to present an N-terminal histidine capable of metal coordination. We exemplify the approach through discovery and characterisation of multiple new metalloprotein families, including two classes termed anglerases for their potential to “fish” transition metals from the bacterial milieu.
Cairo, J.P.L.F., et al. Signal-strapping: a protein-sequence search method for the discovery of metalloproteins. Nature Commun. 2025, 16, 9244. https://doi.org/10.1038/
Bridging a gap in marine sulfur cycling: D-cysteinolic acid degradation pathway discovered
A long-standing puzzle in environmental sulfur cycling is the fate of D-cysteinolic acid, a prominent organosulfur metabolite in aquatic ecosystems. In a new JACS study, we describe a dedicated degradation pathway in the marine model bacterium Ruegeria pomeroyi DSS-3, featuring a PLP-dependent cysteinolic acid racemase (ClaA), an NAD⁺-dependent L-cysteinolic acid dehydrogenase (ClaB) producing L-cysteate, and subsequent conversion to D-cysteate (via CuyB) for cleavage by the sulfolyase CuyA. Bioinformatic analysis indicates homologues are widespread in ocean microbes, with abundance patterns that correlate with surface-water chlorophyll thus linking this pathway to photosynthetic primary production and highlighting D-cysteinolic acid as a key “metabolic currency” in marine microbial networks.
Burchill, L., et al. Bridging a Gap in Marine Sulfur Cycling: Discovery of a D-Cysteinolic Acid Degradation Pathway. J Am Chem Soc. 2025, 147, 47934–47941. https://doi.org/10.1021/jacs.
May 2025
RSC Horizon team Prize video released
 |
Go check out the Chemistry Biology Interface Horizon Prize: Rita and John Cornforth Award video “Uncovering the Chemical Biology of Sulfosugar Metabolism” |

Find out more:
For up-to-date publication details please use the Google Scholar or "full publication list" links below.
Useful links
Research funders include:
![]()


