Dr Federico Sabbadin
Lecturer
Teaching
Teaching
My teaching is driven by my passion for the molecular machines (enzymes) that underpin all life as we know it. My aim is to open the students` eyes to the incredible diversity of shapes and functionalities that Nature has evolved over billions of years, and show them how we can use this knowledge to tackle global challenges.
Tutorials
My tutorials explore and combine two of my favourite topics: enzymes and invertebrates. Ours is truly a planet of the bugs and, among invertebrates, insects stand out. They are the most diverse group of animals, and have boldly colonised virtually all available terrestrial niches. In doing so, they have evolved the most fascinating and bizarre mechanisms to source and digest food, respond to and interact with the environment and other life forms, reproduce, kill each other, socialise and, essentially, survive.
During my tutorials, students explore and critically analyse past and current research on this topic, and appreciate its biotechnological implications towards tackling the great challenges that our society is facing. Students work in groups to deliver presentations on chosen subjects, learn to refine their communications skills, engage in the scientific debate, and receive feedback on their final essay and how to improve their writing skills.
Projects
The offered projects are mostly lab based within CNAP, in line with ongoing key research areas in the group. Students will take part in exciting multidisciplinary investigations into new proteins and enzymes from plant pathogens, algae, and insects, underpinned by omics, data analysis, molecular biology techniques, structural biology and biochemical characterisations.
Research
Overview
Discovering new enzymes for agricultural and industrial applications
Nature hosts a virtually unlimited supply of diverse enzymes that have potential applications in biomass valorisation, food and fuel production, biosynthesis of fine chemicals and pharmaceuticals, crop protection and waste recycling. The Sabbadin Lab uses cutting edge multidisciplinary approaches to identify and characterise these enzymes from plant pathogens, insects and algae.
Microbial virulence factors and crop protection
Microbial pathogens deploy a wide range of enzymes and proteins to weaken and penetrate the plant cell wall, which is the first and most robust protective barrier against infection. Our recent work on the oomycete Phytophthora infestans, the most damaging pathogen of potato and tomato crops in modern agriculture, has led to the discovery of a new family of lytic polysaccharide monooxygenases (LPMOs) and revealed its crucial role during penetration of the plant cell wall by oomycete pathogens (Sabbadin et al., Science, 2021), paving the way to new molecular approaches to fight this devastating disease (Figure 1). We are now expanding our investigations into oomycetes virulence factors using integrated transcriptomics, shotgun proteomics, 3D structural prediction and in vitro biochemical characterisations, to identify key targets for crop protection.

Figure 1. Potato leaves infected with wild type P. infestans (left) and P. infestans stably transformed with plasmid silencing the LPMO coding gene PiAA17C (right).
Insect enzymes for biomass valorisation, food security and biocatalysis
Over 400 million years of evolution, insects have diversified and adapted their proteins and enzymes to efficiently extract nutrients from natural polymers (cellulose, hemicellulose, lignin, waxes, keratin) and detoxify secondary metabolites produced by plants (terpenes, alkaloids) (Figure 2). Our group uses omics, phylogeny, in vitro biochemical characterisation and structural analysis to shed light on the biological roles of these enzymes, and explore their potential use towards plant protection and sustainable conversion of biomass into bioethanol, textiles and new materials.

Figure 2. Insects have evolved enzymes to digest crystalline cellulose (left: firebrats), detoxify terpenes (centre: pine sawflies) and deconstruct keratin (right: clothes moths).
Algal enzymes
Following our discovery of the first family of lytic polysaccharide monooxygenases (LPMOs) in algae (Sabbadin et al. Nature Communications, 2018; Figure 3), we have been exploring the biological roles of these copper-dependent enzymes in algal polysaccharide metabolism. This research is revealing a complex and untapped source of useful enzymes that could help convert abundant algal biomass into food and fuel.
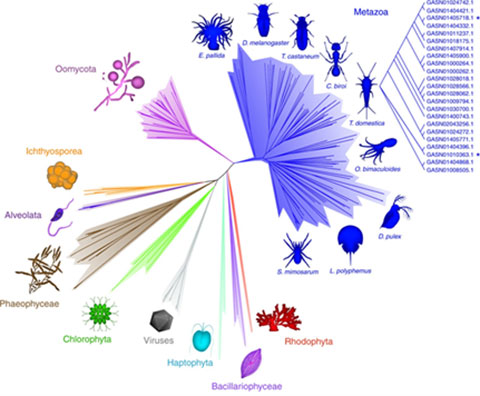
Figure 3. Radial phylogeny of the AA15 LPMO family, the first to encompass animals (metazoan), oomycetes and algae.
.jpg)
